Journal list menu
Export Citations
Download PDFs
Cover Pictures
Cover Picture: By-Product-Free Siloxane-Bond Formation and Programmed One-Pot Oligosiloxane Synthesis (Angew. Chem. Int. Ed. 12/2017)
- Page: 3111
- First Published: 15 February 2017

Oligosiloxanes can be obtained in a single flask in a programmed fashion. In their Communication on page 3168 ff., S. Shimada and co-workers report a novel oligosiloxane synthesis consisting of three successive reactions in one pot: iridium-catalyzed hydrosilylation, boron-catalyzed rearrangement, and boron-catalyzed cross-coupling. The sequence of SiR2 units in the oligosiloxane product can be controlled by simply changing the order of hydrosilane addition.
Inside Cover: Enantioselective Aza-Ene-type Reactions of Enamides with Gold Carbenes Generated from α-Diazoesters (Angew. Chem. Int. Ed. 12/2017)
- Page: 3112
- First Published: 17 February 2017

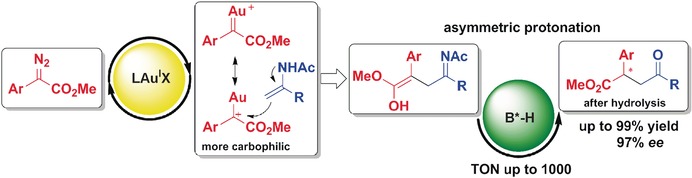
Carbophilic gold carbenes generated by the decomposition of α-diazoesters are highly reactive towards enamides, undergoing an unprecedented aza-ene-type reaction. In their Communication on page 3247 ff., S.-W. Luo, L.-Z. Gong, and co-workers show that the presence of 0.1 mol % of a chiral Brønsted acid is sufficient to obtain synthetically significant γ-keto esters in high yields and with excellent enantioselectivities.
Inside Back Cover: Organoselenium-Catalyzed Regioselective C−H Pyridination of 1,3-Dienes and Alkenes (Angew. Chem. Int. Ed. 12/2017)
- Page: 3393
- First Published: 15 February 2017

A gift from the moon goddesses Selene and Chang'ePyridinium salts are formed in an organoselenium-catalyzed regioselective C−H pyridination of alkenes as described by X. Zhao et al. in their Communication on page 3201 ff. The new pyridinium salts were prepared from 1,3-dienes and fluoropyridinium salts using diselenide catalysts personified as lunar goddesses Selene (Greek) and Chang'e (Chinese).
Back Cover: Facile Synthesis of Polycyclic Pentalenes with Enhanced Hückel Antiaromaticity (Angew. Chem. Int. Ed. 12/2017)
- Page: 3394
- First Published: 24 February 2017

Pentalene with eight π electrons shows pronounced antiaromaticity. When the scaffold is stabilized by fused benzene rings, this structure loses the inherent antiaromaticity. In their Communication on page 3270 ff., H. Oshima, A. Fukazawa, and S. Yamaguchi report a strategy for restoring the lost antiaromaticity in the dibenzo[a,e]pentalene. Fusion with four highly aromatic rings at the peripheral positions gives rise to enhanced antiaromaticity.
Frontispiece
Frontispiece: Ultra-Fast Supercritical Hydrothermal Synthesis of Tobermorite under Thermodynamically Metastable Conditions
- First Published: 10 March 2017

Mineral SynthesisThe durability of buildings—from Roman to modern ones—is partly due to the mineral tobermorite which is used in cement. In their Communication on page 3162 ff., J. J. Gaitero, C. Aymonier et al. report how tobermorite can be synthesized in seconds.
Graphical Abstract
Graphical Abstract: Angew. Chem. Int. Ed. 12/2017
- Pages: 3115-3129
- First Published: 10 March 2017
Corrigendum
Corrigendum: Merging Iron Catalysis and Biocatalysis—Iron Carbonyl Complexes as Efficient Hydrogen Autotransfer Catalysts in Dynamic Kinetic Resolutions
- Page: 3129
- First Published: 10 March 2017
News
Spotlights on our sister journals: Angew. Chem. Int. Ed. 12/2017
- Pages: 3130-3133
- First Published: 10 March 2017
Author Profile
News
Book Reviews
Stereoelectronic Effects. A Bridge between Structure and Reactivity By Igor V. Alabugin.
- Page: 3137
- First Published: 01 February 2017
Highlights
Biocatalysis
The First Biocatalytic Carbon–Silicon Bond Formation
- Pages: 3140-3141
- First Published: 15 February 2017
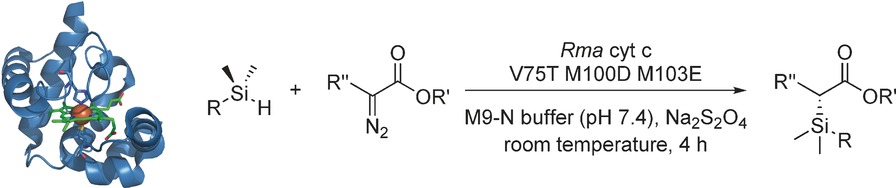
Linking two worlds: As a further expansion of the biocatalytic repertoire, carbene insertion into Si−H bonds catalyzed by the heme protein cytochrome c was recently reported. This new biocatalyst holds great promise because it enables the highly selective incorporation of silicon into molecules without prior protection of existing functional groups.
Reviews
Vesicles
Vesicles in Nature and the Laboratory: Elucidation of Their Biological Properties and Synthesis of Increasingly Complex Synthetic Vesicles
- Pages: 3142-3160
- First Published: 12 October 2016
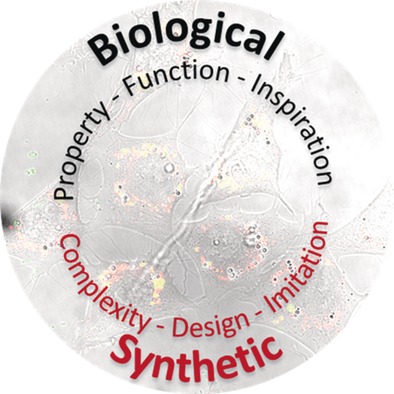
Inspiration–imitation–innovation: Parallel to the elucidation of the biological properties and physiological function of vesicles, increasingly elegant artificial vesicles are being reported. This Review provides an overview of the complex and rapidly developing fields of natural and synthetic vesicles and shows how the two fields can profit from one another.
Communications
Mineral Synthesis | Very Important Paper
Ultra-Fast Supercritical Hydrothermal Synthesis of Tobermorite under Thermodynamically Metastable Conditions
- Pages: 3162-3167
- First Published: 03 February 2017
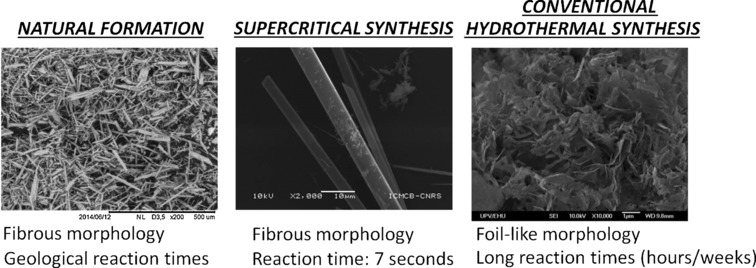
7 Seconds, a geological timescale: The supercritical hydrothermal flow is a new method to produce phases, such as tobermorite, that are metastable under the synthesis conditions. Tobermorite is important in the construction industry. In contrast to traditional methods, reaction/crystallization takes just a few seconds and the product resembles much more closely the natural mineral tobermorite.
Siloxane Synthesis | Very Important Paper
By-Product-Free Siloxane-Bond Formation and Programmed One-Pot Oligosiloxane Synthesis
- Pages: 3168-3171
- First Published: 02 February 2017
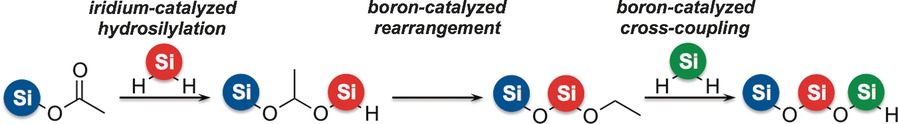
All in one and one for all: A one-pot sequence involving the iridium-catalyzed hydrosilylation of silyl esters and boron-catalyzed rearrangement enabled by-product-free siloxane-bond formation. Oligosiloxanes could also be synthesized in a single flask in a programmed fashion by a sequence of iridium-catalyzed hydrosilylation, boron-catalyzed rearrangement, and boron-catalyzed cross-coupling (see scheme).
C−H Activation
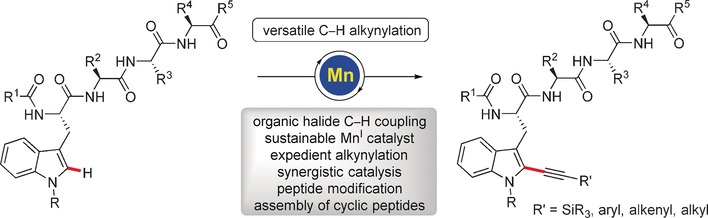
Manganese-Catalyzed C−H Alkynylation: Expedient Peptide Synthesis and Modification
- Pages: 3172-3176
- First Published: 09 February 2017
Bioconjugation | Very Important Paper
Palladium-Mediated Arylation of Lysine in Unprotected Peptides
- Pages: 3177-3181
- First Published: 16 February 2017
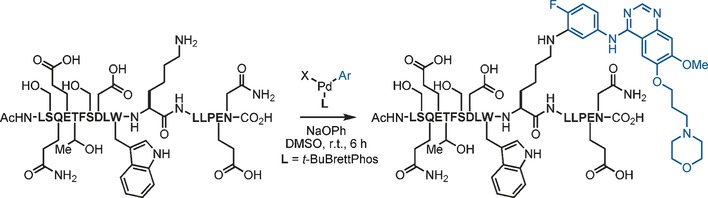
Defenses down: In the presence of a preformed biarylphosphine-supported palladium(II)–aryl complex and a weak base, lysine amino groups in unprotected peptides underwent C−N bond formation at room temperature (see scheme). The process was applicable to the conjugation of a variety of organic compounds, including complex drug molecules, to peptides and was also successfully applied to the formation of cyclic peptides through macrocyclization.
Arenes
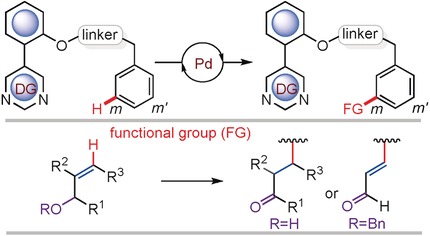
Template-Assisted meta-C−H Alkylation and Alkenylation of Arenes
- Pages: 3182-3186
- First Published: 16 February 2017
Carbon–Heteroatom Coupling
Ni-Catalyzed Stannylation of Aryl Esters via C−O Bond Cleavage
- Pages: 3187-3190
- First Published: 10 February 2017
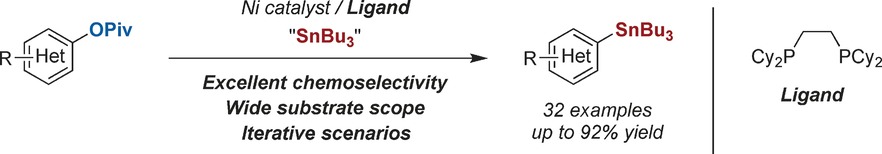
An excellent chemoselectivity profile and a broad substrate scope characterize the title reaction, which contributes to expanding the rather limited portfolio of C−heteroatom bond formations via C−O functionalization. It can be applied to challenging substrate combinations and iteratively, which shows the prospective impact of this protocol in complex settings.
C−H Activation
Synthesis of Active Hexafluoroisopropyl Benzoates through a Hydrogen-Bond-Enabled Palladium(II)-Catalyzed C−H Alkoxycarbonylation Reaction
- Pages: 3191-3195
- First Published: 17 February 2017
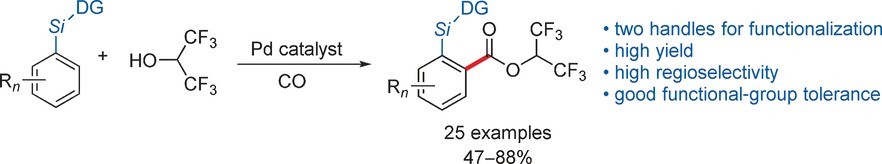
Let's get active: The title reaction of aryl silanes to deliver active hexafluoroisopropyl (HFIP) benzoate esters features high selectivity and good functional-group tolerance. This method yields products bearing two independently modifiable sites. NMR studies reveal the presence of hydrogen bonding between HFIP and a pyrimidine nitrogen atom of the directing group (DG), and it is thought to be crucial for the success of this alkoxycarbonylation reaction.
Organofluorine Chemistry
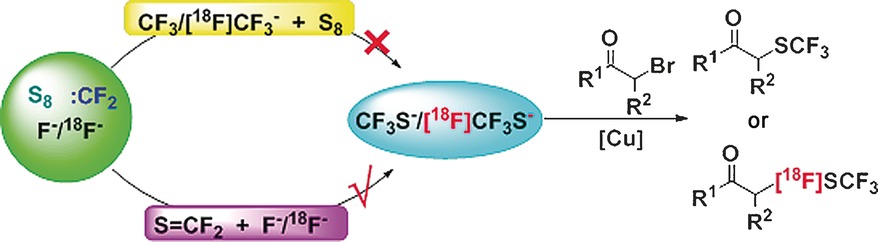
An Unconventional Mechanistic Insight into SCF3 Formation from Difluorocarbene: Preparation of 18F-Labeled α-SCF3 Carbonyl Compounds
- Pages: 3196-3200
- First Published: 14 February 2017
Pyridinium Functionalization
Organoselenium-Catalyzed Regioselective C−H Pyridination of 1,3-Dienes and Alkenes
- Pages: 3201-3205
- First Published: 16 January 2017
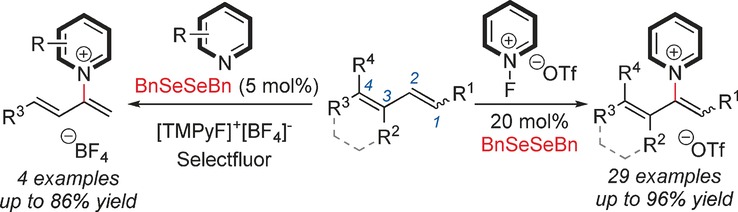
Pyridinium salts are formed in an organoselenium-catalyzed regioselective C−H pyridination of 1,3-dienes. The efficient C-2 selective method was successfully applied to direct C−H pyridination of alkenes. Fluoropyridinium reagents or initially loaded pyridine derivatives acted as pyridine sources in the pyridination reactions.
Carbenes
Efficient Difluoromethylation of Alcohols Using TMSCF2Br as a Unique and Practical Difluorocarbene Reagent under Mild Conditions
- Pages: 3206-3210
- First Published: 09 February 2017
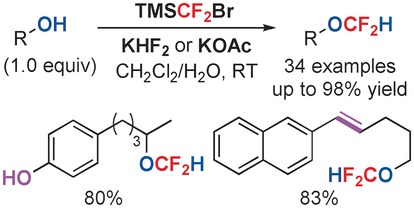
A general method for difluoromethylation of alcohols with difluorocarbene under weakly basic or acidic aqueous conditions by using TMSCF2Br as a unique and practical difluorocarbene reagent is developed. This method is efficient for the selective synthesis of alkyl difluoromethyl ethers from functionalized alcohols, and a mechanism different from difluoromethylation of phenols is disclosed.
Heterobimetallic Complexes
Enhancement of C−H Oxidizing Ability in Co–O2 Complexes through an Isolated Heterobimetallic Oxo Intermediate
- Pages: 3211-3215
- First Published: 14 February 2017
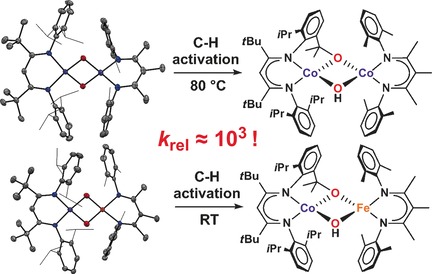
Odd couple: Treatment of a side-on dioxygen complex of cobalt with a low-valent cobalt or iron diketiminate complex affords a homobimetallic Co/Co or a heterobimetallic Fe/Co oxo complex, respectively. C−H activation in the Co/Fe complex is three orders of magnitude faster than in the homobimetallic analogue.
Homogeneous Catalysis
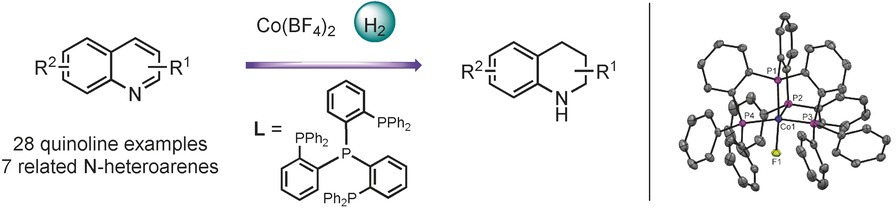
A General and Highly Selective Cobalt-Catalyzed Hydrogenation of N-Heteroarenes under Mild Reaction Conditions
- Pages: 3216-3220
- First Published: 14 February 2017
Cascade Reactions
Palladium-Catalyzed Oxidative Cascade Carbonylative Spirolactonization of Enallenols
- Pages: 3221-3225
- First Published: 17 February 2017
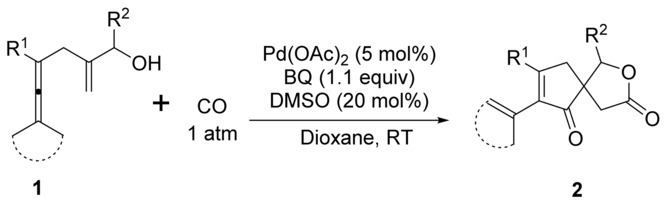
All together now: A highly selective cascade reaction for C−C/C−O bond formation through palladium-catalyzed oxidative carbonylation/carbocyclization/alkoxycarbonylation of enallenols was developed, affording spirolactones bearing an all-carbon quaternary center. Preliminary attempts to obtain enantioselectivity in the carbonylative carbocyclization revealed that the VAPOL-type chiral phosphoric acid serves as a good anionic co-catalyst in this transformation.
Atomic Layer Deposition
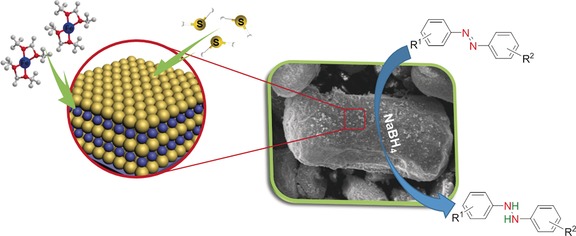
Atomic Layer Deposition of Iron Sulfide and Its Application as a Catalyst in the Hydrogenation of Azobenzenes
- Pages: 3226-3231
- First Published: 07 February 2017
Expanded Porphyrins
Internally 2,5-Thienylene-Bridged [46]Decaphyrin: (Annuleno)annulene Network Consisting of Möbius Aromatic Thia[28]hexaphyrins and Strong Hückel Aromaticity of its Protonated Form
- Pages: 3232-3236
- First Published: 02 March 2017
![Internally 2,5-Thienylene-Bridged [46]Decaphyrin: (Annuleno)annulene Network Consisting of Möbius Aromatic Thia[28]hexaphyrins and Strong Hückel Aromaticity of its Protonated Form](/cms/asset/9755fdcd-fd5e-4453-8c64-9396cea81f3e/anie201700607-toc-0001-m.jpg)
Switching aromaticity: 1,3-Phenylene- and 2,5-thienylene-bridged [46]decaphyrins A and B have been synthesized. While A shows Hückel-aromatic character derived from its global 46π-conjugated circuit, B displays an (annuleno)annulene-type structure consisting of two twisted Möbius-aromatic thia[28]hexaphyrins. Upon protonation, these [46]decaphyrins undergo large structural changes and become strongly aromatic.
Dearomatization | Very Important Paper
Iridium-Catalyzed Intermolecular Asymmetric Dearomatization of β-Naphthols with Allyl Alcohols or Allyl Ethers
- Pages: 3237-3241
- First Published: 09 January 2017
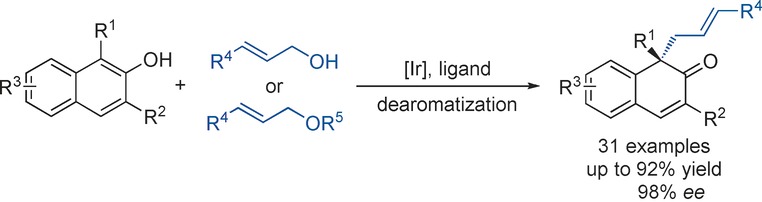
Introducing asymmetry: The iridium catalyst generated from [{Ir(cod)Cl}2] (cod=cyclooctadiene) and a chiral P/olefin ligand facilitates the intermolecular asymmetric dearomatization of β-naphthols with allyl alcohols or allyl ethers. Highly functionalized β-naphthalenone compounds bearing an all-carbon-substituted quaternary chiral center were obtained in up to 92 % yield and 98 % ee.
Allylic Compounds
Enantioselective Dearomatization of Naphthol Derivatives with Allylic Alcohols by Cooperative Iridium and Brønsted Acid Catalysis
- Pages: 3242-3246
- First Published: 14 February 2017
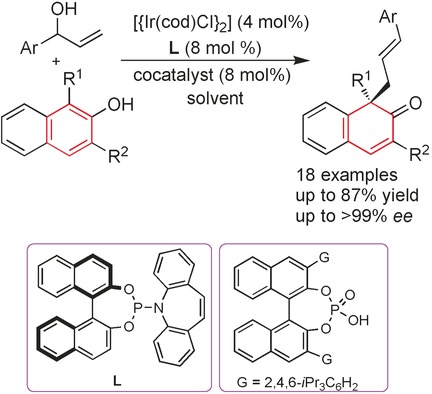
Co-op: The cooperative dual-catalytic system of a chiral iridium complex and phosphoric acid achieves highly enantioselective allylic dearomatization reactions of naphthols with racemic secondary allylic alcohols. The desired β-naphthalenones, bearing an all-carbon quaternary center, were obtained in good yields. Both β-naphthalenone enantiomers can be obtained by simply employing opposite enantiomeric ligands.
Asymmetric Catalysis
Enantioselective Aza-Ene-type Reactions of Enamides with Gold Carbenes Generated from α-Diazoesters
- Pages: 3247-3251
- First Published: 14 February 2017
Self-Assembly
A Two-Tailed Phosphopeptide Crystallizes to Form a Lamellar Structure
- Pages: 3252-3255
- First Published: 13 February 2017
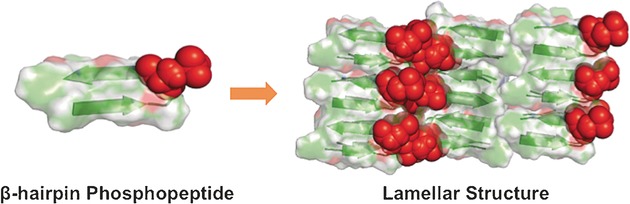
A designed β-hairpin phospholipid-inspired phosphopeptide crystallizes to form a lamellar structure that is stabilized by a variety of hydrogen-bonding interactions. The crystal structure together with ssNMR provide insight into the molecular arrangement within the self-assembled semi-elliptical nanosheets. The crystal structure could prove valuable for enhancing the predictability of intermolecular interactions for the design of self-assembling oligopeptides.
Heterogeneous Catalysis
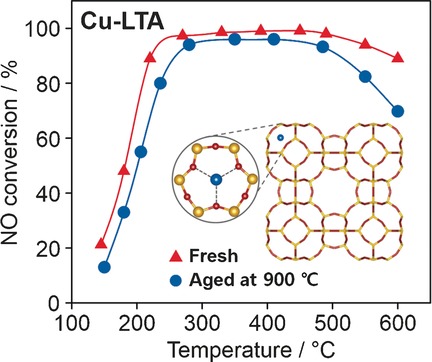
Fully Copper-Exchanged High-Silica LTA Zeolites as Unrivaled Hydrothermally Stable NH3-SCR Catalysts
- Pages: 3256-3260
- First Published: 18 January 2017
Smart Materials | Very Important Paper
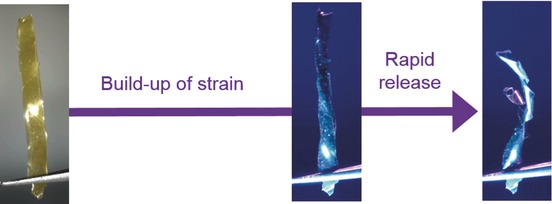
High-Power Actuation from Molecular Photoswitches in Enantiomerically Paired Soft Springs
- Pages: 3261-3265
- First Published: 09 February 2017
C−C Activation
Selective Arene Cleavage by Direct Insertion of Iridium into the Aromatic Ring
- Pages: 3266-3269
- First Published: 17 February 2017
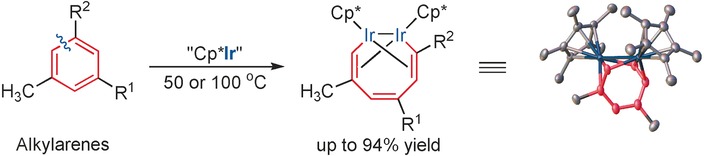
Running mild: A mild and selective insertion of a metal center into the arene ring is reported. The simple Cp*Ir fragment cleaves strong aromatic C−C bonds in alkylarenes without affecting weaker C−H and C−C bonds. This work reveals a conceptually new type of reactivity that could be important for a range of industrial processes involving the generation of chemicals and fuel from coal and plant biomass.
Antiaromaticity
Facile Synthesis of Polycyclic Pentalenes with Enhanced Hückel Antiaromaticity
- Pages: 3270-3274
- First Published: 09 January 2017
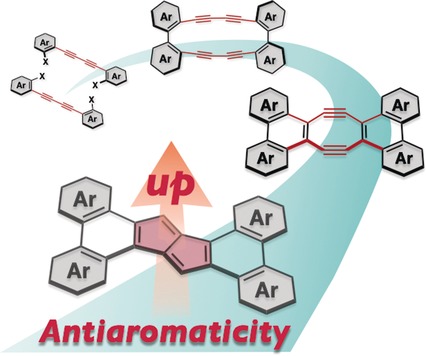
Polycyclic pentalenes with phenanthrene-type π extensions were prepared from 1,4-bis(bromoaryl)-1,3-butadiynes by successive transannular cyclizations of the in situ generated tetrakisdehydro[16]annulenes. These polycyclic pentalenes show not only high thermal stability, but also intriguing absorption and redox properties, due to the pronounced Hückel antiaromaticity.
Boron Cations
Using Ylide Functionalization to Stabilize Boron Cations
- Pages: 3275-3279
- First Published: 10 February 2017
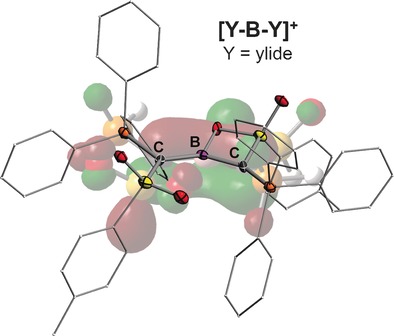
Ylide meets B+: Employing a metalated ylide as donor ligand makes possible the isolation of highly stable ylide-functionalized boron cations. π-Delocalization as well as electrostatic interactions within the C−B−C linkage play the most important role for the high stability of the [Y−B−Y]+ cation and its Lewis base adducts. Reaction with amines results in N−H activation by addition across the B−C bond.
Extended π-Conjugated Systems
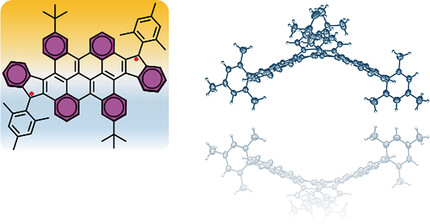
A Stable Saddle-Shaped Polycyclic Hydrocarbon with an Open-Shell Singlet Ground State
- Pages: 3280-3284
- First Published: 15 February 2017
Gels
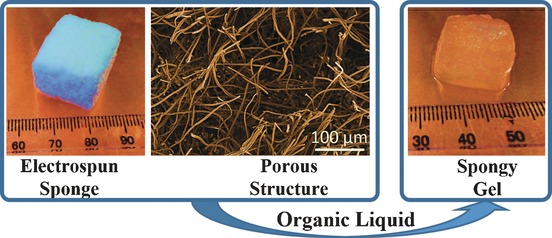
Spongy Gels by a Top-Down Approach from Polymer Fibrous Sponges
- Pages: 3285-3288
- First Published: 14 February 2017
Water Oxidation
Hollow Iron–Vanadium Composite Spheres: A Highly Efficient Iron-Based Water Oxidation Electrocatalyst without the Need for Nickel or Cobalt
- Pages: 3289-3293
- First Published: 14 February 2017
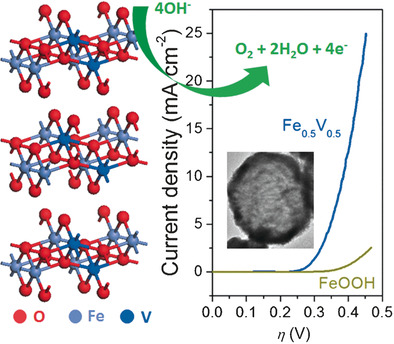
Missing, but not missed: Vanadium-doped FeOOH is a low-cost, highly efficient iron-based electrocatalyst for water oxidation without Ni or Co participation. It exhibits a low overpotential 390 mV (10 mA cm−2 catalytic current density), low Tafel slope of 36.7 mV dec−1, and a considerable durability.
Hybrid Polyoxometalates
Cation Translocation around Single Polyoxometalate–Organic Hybrid Cluster Regulated by Electrostatic and Cation–π Interactions
- Pages: 3294-3298
- First Published: 15 February 2017
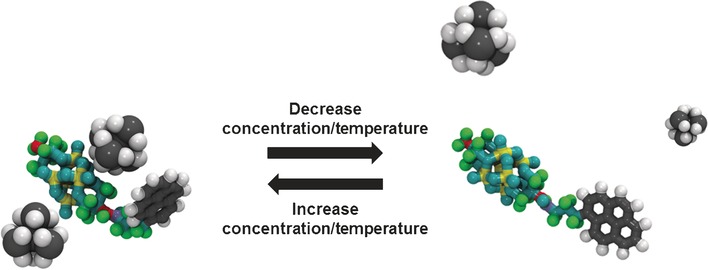
Near and far: Spectroscopic and theoretical studies on the dynamic translocation process of countercations around a polyoxometalate–organic hybrid anionic molecular cluster show that electrostatic interactions and cation–π interactions regulate the position of small countercations around single clusters.
Surface Chemistry
Rapid and Complete Surface Modification with Strain-Promoted Oxidation-Controlled Cyclooctyne-1,2-Quinone Cycloaddition (SPOCQ)
- Pages: 3299-3303
- First Published: 15 February 2017
Organocatalysis
Light-Driven Enantioselective Organocatalytic β-Benzylation of Enals
- Pages: 3304-3308
- First Published: 10 February 2017
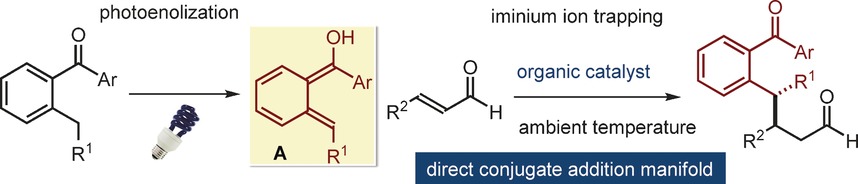
Unconventional Michael: The iminium ion activation of α,β-unsaturated aldehydes allowed stereoselective interception of photochemically generated hydroxy-o-quinodinomethane intermediates (A). The chemistry, which provides a direct entry into enantioenriched β-benzylated aldehydes, proceeds through an unconventional Michael-type addition path instead of a classical cycloaddition manifold. Computational studies have shed light upon the origin of this unexpected reactivity.
Bioorthogonal Chemistry
Steering Siglec–Sialic Acid Interactions on Living Cells using Bioorthogonal Chemistry
- Pages: 3309-3313
- First Published: 14 February 2017
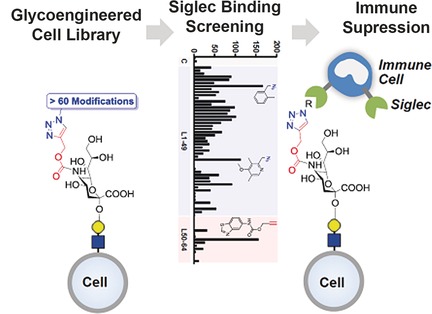
Clicking the immune system off: We report a method to rapidly reprogram the binding of sialic acid sugars on living cells to their cognate Siglec receptors through glycoengineering and click chemistry. Binding could be improved by more than 100-fold and in a selective manner. The modified cells showed potent immunosuppressive activity resulting from strong signaling through Siglecs on immune cells.
Chemoselectivity
Determining the Origin of Rate-Independent Chemoselectivity in CuAAC Reactions: An Alkyne-Specific Shift in Rate-Determining Step
- Pages: 3314-3318
- First Published: 16 February 2017
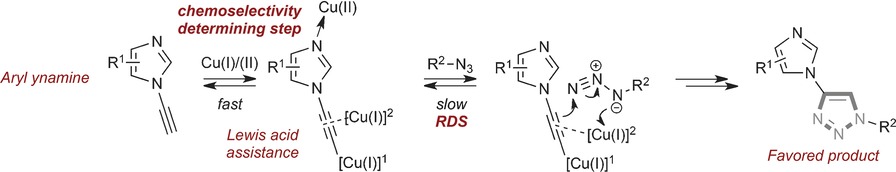
The old switcheroo: A kinetic and spectroscopic analysis of alkyne-dependent chemoselectivity in the copper-catalyzed azide–alkyne click (CuAAC) reaction is reported. Studies of six alkyne subtypes reveal that the rate-determining step (RDS) of an aromatic ynamine class is shifted from acetylide formation to the azide ligation/migratory insertion event allowing chemoselectivity independent of overall rate.
Cross-Coupling Reactions
Alkyl−(Hetero)Aryl Bond Formation via Decarboxylative Cross-Coupling: A Systematic Analysis
- Pages: 3319-3323
- First Published: 10 February 2017
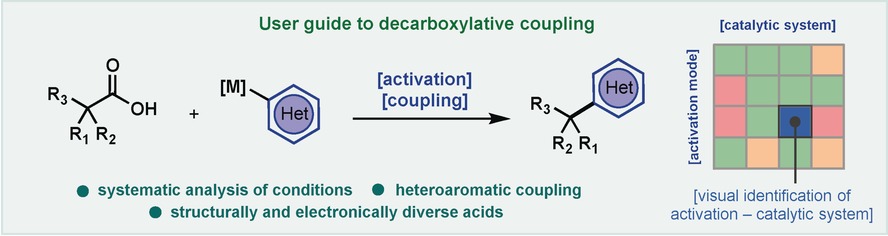
Cross-coupling meets amide bond formation: Decarboxylative cross-coupling with redox-active esters forms C−C bonds using boronic acids, organozinc, and organomagnesium species with simplicity comparable to amide bond formation. The extensive study enables selection of optimal activating agents and conditions across various substrates, including notoriously challenging heteroarenes.
Protein Chemistry
Inversion of the Side-Chain Stereochemistry of Indvidual Thr or Ile Residues in a Protein Molecule: Impact on the Folding, Stability, and Structure of the ShK Toxin
- Pages: 3324-3328
- First Published: 14 February 2017
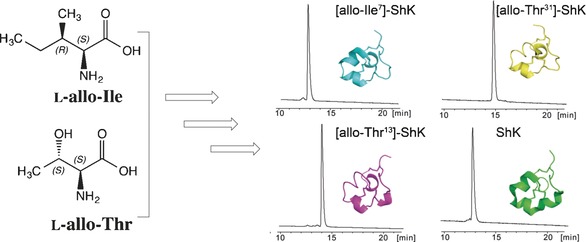
Ch-ch-ch-changes: Guided by molecular dynamics calculations, chemical protein synthesis was used to explore the impact of allo-Thr and allo-Ile substitutions for individual Thr and Ile residues on the folding, crystal structure, and stability of the ShK protein. The experimental folding and thermal stability data matched well with the computational results.
Artificial Photosynthesis
A Ruthenium Complex–Porphyrin–Fullerene-Linked Molecular Pentad as an Integrative Photosynthetic Model
- Pages: 3329-3333
- First Published: 14 February 2017
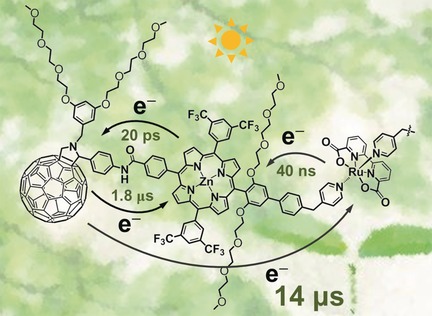
Pent-up energy: A ruthenium-based water oxidation catalyst linked to porphyrin–fullerene units in a molecular pentad has been synthesized. Transient absorption indicated the photoinduced multistep electron transfer afforded a long-lived hole–electron pair, and photocatalytic water oxidation by the pentad was achieved in the presence of sacrificial oxidant.
Asymmetric Synthesis
Synthesis of Enantiopure C3-Symmetric Triangular Molecules
- Pages: 3334-3338
- First Published: 14 February 2017
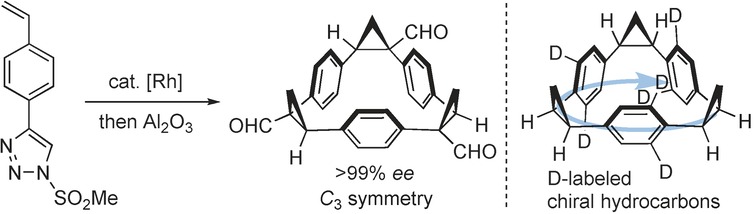
My hat, it has three corners: Enantiopure C3-symmetric triangular macrocycles were synthesized by means of triple asymmetric cyclopropanation. This method is successfully extended to the synthesis of an enantiopure hydrocarbon, which owes its chirality to asymmetric distribution of H/D atoms on the benzene rings.
Molecularly Imprinted Polymers
Enzyme-Initiated Free-Radical Polymerization of Molecularly Imprinted Polymer Nanogels on a Solid Phase with an Immobilized Radical Source
- Pages: 3339-3343
- First Published: 14 February 2017
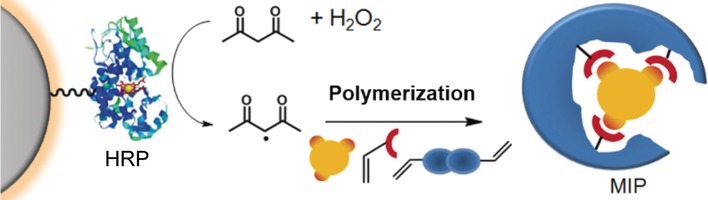
Mighty Immobilized Peroxidase: The radical polymerization of methacrylate or vinyl monomers and cross-linkers was initiated by immobilized horseradish peroxidase (HRP) to prepare molecularly imprinted polymer (MIP) nanogels in aqueous media (see scheme). MIP nanoparticles with sizes between 50 and 300 nm were obtained with good binding properties and high selectivity for the target molecule, the herbicide 2,4-dichlorophenoxyacetic acid.
Macrocyclization
Intramolecular Amido Transfer Leading to Structurally Diverse Nitrogen-Containing Macrocycles
- Pages: 3344-3348
- First Published: 10 February 2017
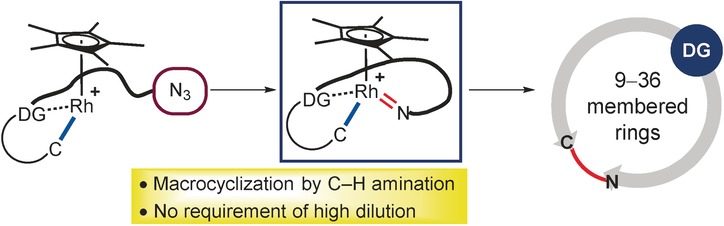
Azamacrocycles: A rhodium-catalyzed inner-sphere intramolecular C−H amination of tailormade acetophenone ketoximes tethered with either aryl or alkyl azides furnishes azamacrocyclic compounds with up to 36-membered rings. While substrates bearing aryl azides underwent a monomeric ring formation in high yields, a dimeric double cyclization took place exclusively with alkyl-azide-tethered ketoximes.
Foldamers
Reversible Stereoselective Folding/Unfolding Fueled by the Interplay of Photoisomerism and Hydrogen Bonding
- Pages: 3349-3353
- First Published: 14 February 2017
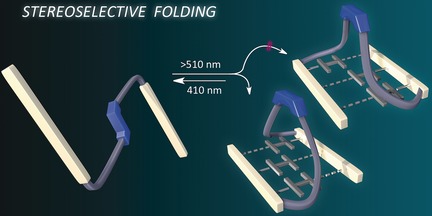
Know when to fold 'em: A linear molecular architecture equipped with complementary three-fold hydrogen bonding units embedded with a photoswitchable trans-tetrafluoroazobenzene moiety was synthesized. The trans to cis photoisomerism of the azobenzene unit induced changes in the molecular architecture as a result of intramolecular hydrogen bonding as evidenced by NMR spectroscopy and size exclusion chromatography.
Cross-Coupling | Very Important Paper
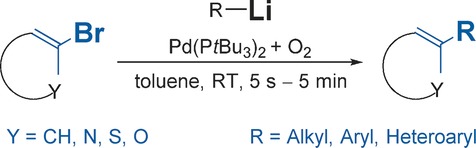
Oxygen Activated, Palladium Nanoparticle Catalyzed, Ultrafast Cross-Coupling of Organolithium Reagents
- Pages: 3354-3359
- First Published: 14 February 2017
Electron Donors
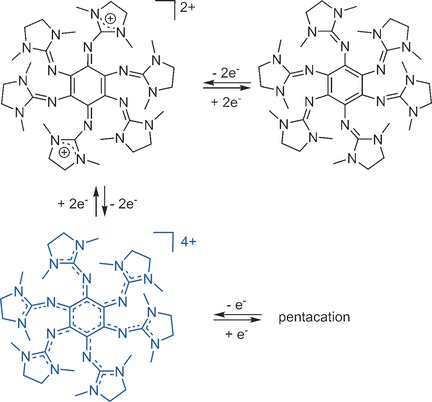
A Stable Hexakis(guanidino)benzene: Realization of the Strongest Neutral Organic Four-Electron Donor
- Pages: 3360-3363
- First Published: 17 February 2017
Gold Catalysis
On the Gold-Catalyzed Generation of Vinyl Cations from 1,5-Diynes
- Pages: 3364-3368
- First Published: 14 February 2017
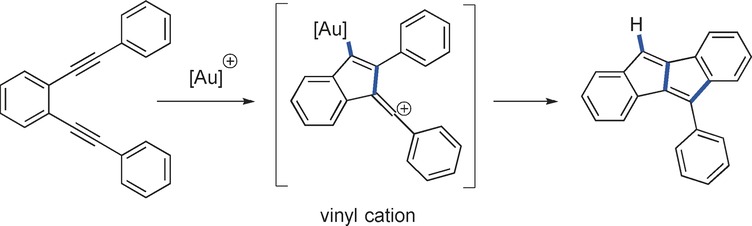
Positive intermediates: The combination of non-terminal 1,5-diynes with cationic gold complexes enables the generation of highly reactive vinyl cations that can be used for the synthesis of unsymmetrically substituted dibenzopentalenes. Quantum-chemical calculations indicate a fast valence tautomer equilibrium between a gold alkyne complex and the vinyl cation.
Protein Spectroscopy
Solid-state NMR and EPR Spectroscopy of Mn2+-Substituted ATP-Fueled Protein Engines
- Pages: 3369-3373
- First Published: 13 February 2017
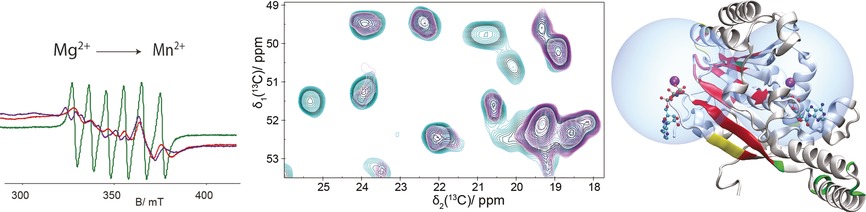
Metal location: Both EPR and solid-state NMR spectra change on the substitution of Mg2+ by Mn2+ in two different ATP:Mg2+ fueled protein engines. While EPR spectra report metal binding and metal-center geometry, NMR paramagnetic relaxation enhancements localize residues at the binding site. Both techniques indicate the location of paramagnetic ions in proteins.
Double Helicenes
Benzo-Fused Double [7]Carbohelicene: Synthesis, Structures, and Physicochemical Properties
- Pages: 3374-3378
- First Published: 14 December 2016
![Benzo-Fused Double [7]Carbohelicene: Synthesis, Structures, and Physicochemical Properties](/cms/asset/5afd2b4a-0e05-4400-86a9-c4c2a80c4491/anie201610434-toc-0001-m.jpg)
Carbon-rich compounds: An all-carbon double [7]helicene has been synthesized by regioselective cyclodehydrogenation. The compound represents the highest homolog of the double carbohelicenes. The twisted conformers D7H-1 and D7H-2 were separated by recrystallization, and their double helicene structures were elucidated by X-ray crystallography.
Condensed polycycles
A Naphtho-Fused Double [7]Helicene from a Maleate-Bridged Chrysene Trimer
- Pages: 3379-3382
- First Published: 09 February 2017
Natural Product Synthesis
First Stereoselective Total Synthesis of a Dimeric Naphthoquinonopyrano-γ-lactone: (+)-γ-Actinorhodin
- Pages: 3383-3388
- First Published: 17 February 2017
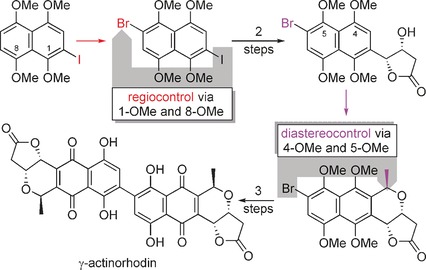
Pairs of peri MeO groups as mediators allowed seemingly distant Br or I substituents to exert regio- and diastereocontrol during naphthalene functionalization by electrophilic aromatic substitution. A bromonaphthalene-fused pyrano-γ-lactone resulted, which was dimerized in a Suzuki coupling. Removal of the methoxy groups and oxidation gave isomerically pure (+)-γ-actinorhodin in eleven steps.
Deoxygenation
B(C6F5)3-Catalyzed Chemoselective Defunctionalization of Ether-Containing Primary Alkyl Tosylates with Hydrosilanes
- Pages: 3389-3391
- First Published: 09 February 2017
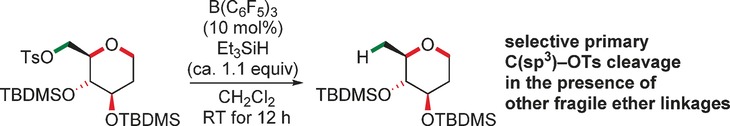
The weakest link: Hydrosilanes activated by B(C6F5)3 are known to cleave ethers as well as silyl ethers. However, more reactive tosylates are cleaved prior to those ethers, which allows for the chemoselective deoxygenation of diols and polyols. Other functional groups such as carboxyl groups are also tolerated. Defunctionalization of phenethyl tosylate motifs proceeds with anchimeric assistance (rearrangement).









![A Naphtho-Fused Double [7]Helicene from a Maleate-Bridged Chrysene Trimer](/cms/asset/5ba3f90a-d23c-434a-885c-73f0730b8d72/anie201610793-toc-0001-m.jpg)