Palladium-Catalyzed Oxidative Cascade Carbonylative Spirolactonization of Enallenols
Dr. Youai Qiu
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 10691 Stockholm, Sweden
Search for more papers by this authorBin Yang
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 10691 Stockholm, Sweden
Search for more papers by this authorDr. Tuo Jiang
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 10691 Stockholm, Sweden
Search for more papers by this authorDr. Can Zhu
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 10691 Stockholm, Sweden
Search for more papers by this authorCorresponding Author
Prof. Dr. Jan-E. Bäckvall
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 10691 Stockholm, Sweden
Search for more papers by this authorDr. Youai Qiu
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 10691 Stockholm, Sweden
Search for more papers by this authorBin Yang
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 10691 Stockholm, Sweden
Search for more papers by this authorDr. Tuo Jiang
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 10691 Stockholm, Sweden
Search for more papers by this authorDr. Can Zhu
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 10691 Stockholm, Sweden
Search for more papers by this authorCorresponding Author
Prof. Dr. Jan-E. Bäckvall
Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 10691 Stockholm, Sweden
Search for more papers by this authorGraphical Abstract
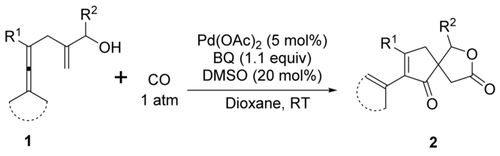
All together now: A highly selective cascade reaction for C−C/C−O bond formation through palladium-catalyzed oxidative carbonylation/carbocyclization/alkoxycarbonylation of enallenols was developed, affording spirolactones bearing an all-carbon quaternary center. Preliminary attempts to obtain enantioselectivity in the carbonylative carbocyclization revealed that the VAPOL-type chiral phosphoric acid serves as a good anionic co-catalyst in this transformation.
Abstract
A highly selective palladium-catalyzed oxidative carbonylation/carbocyclization/alkoxycarbonylation of enallenols to afford spirolactones bearing an all-carbon quaternary center was developed. This transformation involves the overall formation of three C−C bonds and one C−O bond through a cascade insertion of carbon monoxide (CO), an olefin, and CO. Preliminary experiments on chiral anion-induced enantioselective carbonylation/carbocyclization of enallenols afforded spirolactones with moderate enantioselectivity.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201612384-sup-0001-misc_information.pdf4.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected examples involving palladium-catalyzed oxidative carbocyclization of allenes:
- 1aA. K. Å. Persson, J.-E. Bäckvall, Angew. Chem. Int. Ed. 2010, 49, 4624; Angew. Chem. 2010, 122, 4728;
- 1bA. K. Å. Persson, T. Jiang, M. Johnson, J.-E. Bäckvall, Angew. Chem. Int. Ed. 2011, 50, 6155; Angew. Chem. 2011, 123, 6279;
- 1cY. Deng, T. Bartholomeyzik, A. K. Å. Persson, J. Sun, J.-E. Bäckvall, Angew. Chem. Int. Ed. 2012, 51, 2703; Angew. Chem. 2012, 124, 2757.
- 2Selected reviews involving palladium oxidative carbocyclization:
- 2aE. M. Beccalli, G. Broggini, M. Martinelli, S. Sottocornola, Chem. Rev. 2007, 107, 5318;
- 2bF. Dénès, A. Pérez-Luna, F. Chemla, Chem. Rev. 2010, 110, 2366;
- 2cC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215;
- 2dY. Deng, A. K. Å. Persson, J.-E. Bäckvall, Chem. Eur. J. 2012, 18, 11498.
- 3Selected examples involving palladium-catalyzed oxidative carbocyclization:
- 3aT. Wu, X. Xu, G. Liu, Angew. Chem. Int. Ed. 2011, 50, 12578; Angew. Chem. 2011, 123, 12786;
- 3bX. Xu, T. Wu, H. Wang, Y. Guo, G. Liu, J. Am. Chem. Soc. 2012, 134, 878;
- 3cR. Zhu, S. L. Buchwald, Angew. Chem. Int. Ed. 2012, 51, 1926; Angew. Chem. 2012, 124, 1962;
- 3dS. Jaegli, J. Dufour, H. Wei, T. Piou, X. Duan, J. Vors, L. Neuville, J. Zhu, Org. Lett. 2010, 12, 4498.
- 4
- 4aC. M. R. Volla, J. Mazuela, J.-E. Bäckvall, Chem. Eur. J. 2014, 20, 7608;
- 4bM. R. Volla, J. Mazuela, J.-E. Bäckvall, Org. Lett. 2014, 16, 4174;
- 4cC. Zhu, B. Yang, J.-E. Bäckvall, J. Am. Chem. Soc. 2015, 137, 11868;
- 4dY. Qiu, B. Yang, C. Zhu, J.-E. Bäckvall, Chem. Sci. 2017, 8, 616;
- 4eC. Zhu, B. Yang, Y. Qiu, J.-E. Bäckvall; Angew. Chem. Int. Ed. 2016, 55, 14405. Angew.Chem. 2016, 128, 14617; Angew. Chem. Int. Ed. 2016, 55, 14405. Angew.Chem. 2016, 128, 14617.
- 5Selected reviews on palladium-catalyzed carbonylation:
- 5aA. Brennführer, H. Neumann, M. Beller, Angew. Chem. Int. Ed. 2009, 48, 4114; Angew. Chem. 2009, 121, 4176;
- 5bX.-F. Wu, H. Neumann, M. Beller, Chem. Rev. 2013, 113, 1.
- 6
- 6aY. Fan, H. Zhang, Y. Zhou, H. Liu, W. Tang, B. Zhou, J. Zuo, J. Yue, J. Am. Chem. Soc. 2015, 137, 138;
- 6bH. Oh, J. B. Gloer, C. A. Shearer, J. Nat. Prod. 1999, 62, 497;
- 6cM. Massias, S. Rebuffat, L. Molho, A. Chiaroni, C. Riche, B. Bodo, J. Am. Chem. Soc. 1990, 112, 8112;
- 6dD. B. Stierle, A. A. Stierle, T. Bugni, J. Org. Chem. 2003, 68, 4966.
- 7Selected reviews:
- 7aR. Rios, Chem. Soc. Rev. 2012, 41, 1060;
- 7bS. Kotha, A. Deb, K. Lahiri, E. Manivannan, Synthesis 2009, 165;
- 7cV. A. D′yakonov, O. A. Trapeznikova, A. de Meijere, U. M. Dzhemilev, Chem. Rev. 2014, 114, 5775.
- 8
- 8aJ. Christoffers, A. Baro, Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis, Wiley-VCH, Weinheim, 2005;
- 8bA. Steven, L. E. Overman, Angew. Chem. Int. Ed. 2007, 46, 5488; Angew. Chem. 2007, 119, 5584;
- 8cZ. Chen, J. Sun, Angew. Chem. Int. Ed. 2013, 52, 13593; Angew. Chem. 2013, 125, 13838;
- 8dZ. Chen, X. Duan, P. Zhou, S. Ali, J. Luo, Y. Liang, Angew. Chem. Int. Ed. 2012, 51, 1370; Angew. Chem. 2012, 124, 1399;
- 8eJ. P. Das, H. Chechik, I. Marek, Nat. Chem. 2009, 1, 128.
- 9Selected reviews:
- 9aA. Fürstner, Chem. Soc. Rev. 2009, 38, 3208;
- 9bL. Fensterbank, M. Malacria, Acc. Chem. Res. 2014, 47, 953;
- 9cM. N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 2014, 510, 485;
- 9dJ. Mahatthananchai, J. W. Bode, Acc. Chem. Res. 2014, 47, 696;
- 9eD. T. Cohen, K. A. Scheidt, Chem. Sci. 2012, 3, 53;
- 9fA. Grossmann, D. Enders, Angew. Chem. Int. Ed. 2012, 51, 314; Angew. Chem. 2012, 124, 320.
- 10
- 10aC. Burstein, F. Glorius, Angew. Chem. Int. Ed. 2004, 43, 6205; Angew. Chem. 2004, 116, 6331;
- 10bS. S. Sohn, E. L. Rosen, J. W. Bode, J. Am. Chem. Soc. 2004, 126, 14370.
- 11Selected examples:
- 11aJ. Dugal-Tessier, E. A. O′Bryan, T. B. H. Schroeder, D. T. Cohen, K. A. Scheidt, Angew. Chem. Int. Ed. 2012, 51, 4963; Angew. Chem. 2012, 124, 5047;
- 11bC. Guo, M. Schedler, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2014, 53, 10232; Angew. Chem. 2014, 126, 10397;
- 11cA. Patra, A. Bhunia, S. R. Yetra, R. G. Gonnade, A. T. Biju, Org. Chem. Front. 2015, 2, 1584;
- 11dH. H. Liao, A. Chatupheeraphat, C. C. Hsiao, I. Atodiresei, M. Rueping, Angew. Chem. Int. Ed. 2015, 54, 15540; Angew. Chem. 2015, 127, 15760.
- 12Selected reviews:
- 12aC. Zhuo, W. Zhang, S. You, Angew. Chem. Int. Ed. 2012, 51, 12662; Angew. Chem. 2012, 124, 12834;
- 12bQ. Chen, Z. Ye, Y. Duan, Y. Zhou, Chem. Soc. Rev. 2013, 42, 497;
- 12cQ. Ding, Y. Ye, R. Fan, Synthesis 2013, 1;
- 12dW. Zi, Z. Zuo, D. Ma, Acc. Chem. Res. 2015, 48, 702.
- 13Selected examples:
- 13aQ. Yin, S. You, Chem. Sci. 2011, 2, 1344;
- 13bB. Hu, Y. Li, W. Dong, K. Ren, X. Xie, J. Wan, Z. Zhang, Chem. Commun. 2016, 52, 3709;
- 13cB. S. Matsuura, A. G. Condie, R. C. Buff, G. J. Karahalis, C. R. J. Stephenson, Org. Lett. 2011, 13, 6320;
- 13dW. Wu, R. Xu, L. Zhang, S. You, Chem. Sci. 2016, 7, 3427.
- 14Selected examples:
- 14aS. Kotha, K. Mandal, Tetrahedron Lett. 2004, 45, 1391;
- 14bS. Kotha, K. Mandal, A. Tiwari, S. M. Mobin, Chem. Eur. J. 2006, 12, 8024;
- 14cN. L. Brock, J. S. Dickschat, Eur. J. Org. Chem. 2011, 5167;
- 14dD. E. White, I. C. Stewart, R. H. Grubbs, B. M. Stoltz, J. Am. Chem. Soc. 2008, 130, 810.
- 15Selected examples:
- 15aE. M. Ferreira, B. M. Stoltz, J. Am. Chem. Soc. 2003, 125, 9578;
- 15bH. Zhang, E. M. Ferreira, B. M. Stoltz, Angew. Chem. Int. Ed. 2004, 43, 6144; Angew. Chem. 2004, 116, 6270;
- 15cE. M. Ferreira, H. Zhang, B. M. Stoltz, Tetrahedron 2008, 64, 5987;
- 15dM. M. Abelman, L. E. Overman, J. Am. Chem. Soc. 1988, 110, 2328;
- 15eM. Pérez-Gómez, J. A. García-López, Angew. Chem. Int. Ed. 2016, 55, 14389; Angew. Chem. 2016, 128, 14601;
- 15fH. Yoon, A. Lossouarn, F. Landau, M. Lautens, Org. Lett. 2016, 18, 6324.
- 16Y. Qiu, B. Yang, C. Zhu, J.-E. Bäckvall, J. Am. Chem. Soc. 2016, 138, 13846.
- 17Formation of oxaspirolactone through palladium-catalyzed carbonylative spirolactonization of hydroxycyclopropanols: D. C. Davis, K. L. Walker, C. Hu, R. N. Zare, R. M. Waymouth, M. Dai, J. Am. Chem. Soc. 2016, 138, 10693.
- 18Selected examples for formation of spirolactones:
- 18aL. Su, L. Shen, S. Ye, Chem. Comnun. 2011, 47, 10136;
- 18bL. Gala, A. Mendoza, F. J. Fananas, F. Rodriguez, Chem. Comnun. 2013, 49, 2715;
- 18cM. A. Bigi, S. A. Reed, M. C. White, J. Am. Chem. Soc. 2012, 134, 9721;
- 18dZ. Xiao, C. Yu, T. Li, X.-S. Wang, C. Yao, Org. Lett. 2014, 16, 3632;
- 18eJ. Li, B. Sahoo, C. G. Daniliuc, F. Glorius, Angew. Chem. Int. Ed. 2014, 53, 10515; Angew. Chem. 2014, 126, 10683.
- 19
- 19aC. Zhu, B. Yang, T. Jiang, J.-E. Bäckvall, Angew. Chem. Int. Ed. 2015, 54, 9066; Angew. Chem. 2015, 127, 9194;
- 19bY. Qiu, B. Yang, C. Zhu, J.-E. Bäckvall, Angew. Chem. Int. Ed. 2016, 55, 6520; Angew. Chem. 2016, 128, 6630.
- 20Selected reviews:
- 20aM. Mahlau, B. List, Angew. Chem. Int. Ed. 2013, 52, 518; Angew. Chem. 2013, 125, 540;
- 20bR. J. Phipps, G. L. Hamilton, F. D. Toste, Nat. Chem. 2012, 4, 603;
- 20cF. Parmar, E. Sugiono, S. Raja, M. Rueping, Chem. Rev. 2014, 114, 9047;
- 20dD. Chen, Z. Han, X. Zhou, L. Gong, Acc. Chem. Res. 2014, 47, 2365.
- 21Selected examples on the use of chiral phosphoric acids:
- 21aT. Jiang, T. Bartholomeyzik, J. Mazuela, J. Willersinn, J.-E. Bäckvall, Angew. Chem. Int. Ed. 2015, 54, 6024; Angew. Chem. 2015, 127, 6122;
- 21bG. Jiang, B. List, Angew. Chem. Int. Ed. 2011, 50, 9471; Angew. Chem. 2011, 123, 9643;
- 21cK. Ohmatsu, N. Imagawa, T. Ooi, Nat. Chem. 2014, 6, 47;
- 21dP. Wang, H. Lin, Y. Zhai, Z. Han, L. Gong, Angew. Chem. Int. Ed. 2014, 53, 12218; Angew. Chem. 2014, 126, 12414;
- 21eZ. Chai, T. J. Rainey, J. Am. Chem. Soc. 2012, 134, 3615.
- 22
- 22aJ. Bao, W. D. Wulff, J. B. Dominy, M. J. Fumo, E. B. Grant, A. C. Rob, M. C. Whitcomb, S.-M. Yeung, R. L. Ostrander, A. L. Rheingold, J. Am. Chem. Soc. 1996, 118, 3392;
- 22bZ. Ding, W. E. G. Osminski, H. Ren, W. D. Wulff, Org. Process Res. Dev. 2011, 15, 1089;
- 22cA. A. Desai, W. D. Wulff, Synthesis 2010, 21, 3670;
- 22dB. Yang, Y. Qiu, T. Jiang, W. D. Wulff, X. Yin, C. Zhu, J.-E. Bäckvall, Angew. Chem. Int. Ed. 2017, DOI: 10.1002/anie.201612385; Angew. Chem. 2017, DOI: 10.1002/ange.201612385.
- 23For details of kinetic isotope effect study, see the Supporting Information.