Journal list menu
Export Citations
Download PDFs
Review
The Ozone Layer
Climate Change and Atmospheric Chemistry: How Will the Stratospheric Ozone Layer Develop?
- First Published: 04 October 2010
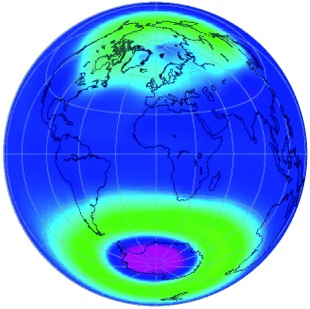
Since the industrial revolution began about 150 years ago, the concentration of greenhouse gases such as CO2 in the atmosphere has increased dramatically, with corresponding consequences for the climate. For over 25 years, destruction of the ozone layer (pink and green regions on the globe), which is caused by chlorofluorocarbons, has also been observed. The future development of the ozone layer and of the climate are closely related to each other.
Nanoparticle Synthesis
Thermodynamics versus Kinetics in Nanosynthesis
- First Published: 23 December 2014
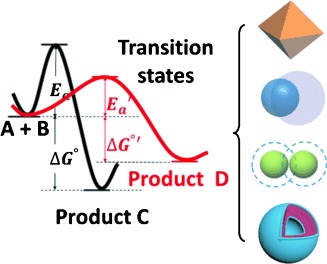
Understanding starts with distinction: Distinguishing between the thermodynamically and kinetically controlled scenarios is of critical importance when analyzing the complex phenomena in nanosynthesis, such as the growth of nanoparticles, their aggregation, and the shape evolution of polymer nanostructures. The processes are examined in detail in this Review and the mechanistic proposals are categorized in the common framework of thermodynamics and kinetics.
Communications
In Situ Spectroscopy
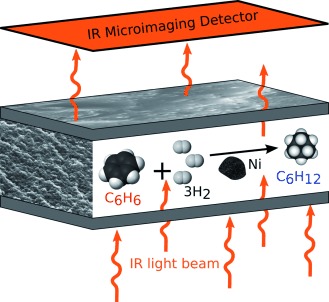
Microimaging of Transient Concentration Profiles of Reactant and Product Molecules during Catalytic Conversion in Nanoporous Materials†
- First Published: 26 February 2015
Heterogeneous Catalysis
Two-Dimensional Spatial Resolution of Concentration Profiles in Catalytic Reactors by Planar Laser-Induced Fluorescence: NO Reduction over Diesel Oxidation Catalysts†
- First Published: 16 January 2015
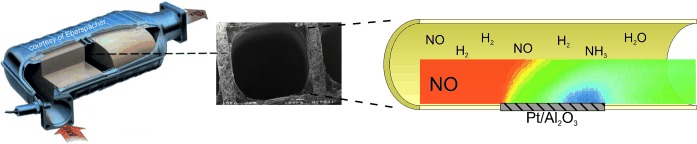
The measurement of two-dimensional species concentration profiles in the gas phase over catalytic walls is achieved by planar laser-induced fluorescence. The interaction of catalytic surface kinetics and mass transport is exemplarily studied for the reduction of NO by hydrogen to ammonia over a diesel oxidation catalyst with platinum as an active component.
Reviews
Sodium Ion Batteries
The Emerging Chemistry of Sodium Ion Batteries for Electrochemical Energy Storage
- First Published: 04 February 2015
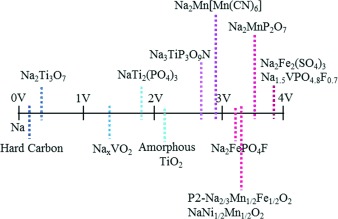
Below lithium: Concerns over the future cost and sustainability of resources of lithium has led to a global trend to develop low-cost sodium-ion batteries. Central to this has been the fast-developing field of non-aqueous batteries that could employ a plethora of materials for the positive and negative electrodes, and electrolytes. Apart from sustainability, they offer structural and electrochemical benefits compared to their Li analogues.
Communications
Organocatalysis
Rationalization of an Unusual Solvent-Induced Inversion of Enantiomeric Excess in Organocatalytic Selenylation of Aldehydes†
- First Published: 07 July 2014
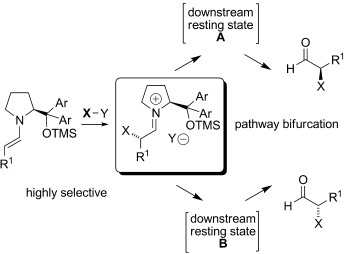
Off the beaten path: Studies of an unusual inversion of the sense of enantioselectivity for the selenylation of aldehydes catalyzed by a diphenylprolinol ether catalyst provides support for a mechanistic concept beyond the simple steric model developed for enamine catalysis in these systems. A general role for downstream intermediates in selectivity outcomes in organocatalysis is discussed. TMS=trimethylsilyl.
CO Oxidation
Catalytically Active and Spectator Ce3+ in Ceria-Supported Metal Catalysts†
- First Published: 09 June 2015
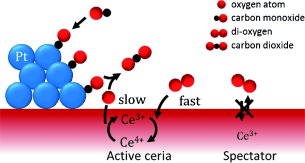
Short-lived Ce3+ is the active and kinetically relevant intermediate during catalytic CO oxidation on a ceria-supported metal catalyst, whereas long-lived Ce3+ species are inactive spectators. This was shown by in situ resonant X-ray emission spectroscopy and quantitative correlation between the initial rate of Ce3+ formation under transient conditions and the overall CO oxidation rate.
Gas-Phase Chemistry
Iron-Promoted CC Bond Formation in the Gas Phase
- First Published: 08 October 2015
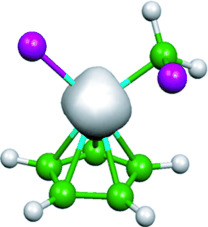
Ion–molecule reactions: A remarkable reorganization occured in iron-containing cations under ambient conditions. Fast and effective carbon–chlorine activation and carbon–carbon coupling was observed in gas-phase mixtures containing ferrocene and dichloromethane, through reaction intermediates (see picture) that eventually extrude the iron giving ferrous chloride.
Mechanisms of Mechanochemistry
Laboratory Real-Time and In Situ Monitoring of Mechanochemical Milling Reactions by Raman Spectroscopy†
- First Published: 24 April 2014
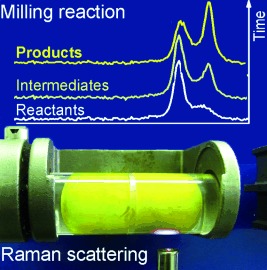
Out of the dark: Mechanochemical reaction mechanisms were studied by a laboratory Raman spectroscopy technique developed for in situ and real-time monitoring of milling reactions (see picture). The technique enabled the course of mechanochemical transformations of coordination polymers and organic materials to be followed as well as the study of liquid additives.
Heterogeneous Catalysis
Oxidative Dehydrogenation on Nanocarbon: Intrinsic Catalytic Activity and Structure–Function Relationships
- First Published: 21 September 2015
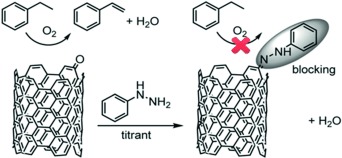
Active duty: An in situ titration process, using phenyl hydrazine as the titrant, is employed to quantify the number of active sites on nanocarbon catalysts for alkane oxidative dehydrogenation reactions. Using this method, the turnover frequency for conversion of the ethyl benzene substrate is used as a reliable kinetic parameter to evaluate the intrinsic activity of the nanocarbon catalysts.
Reaction Mechanisms
Concerted Ring Opening and Cycloaddition of Chiral Epoxy Enolsilanes with Dienes†
- First Published: 07 May 2015
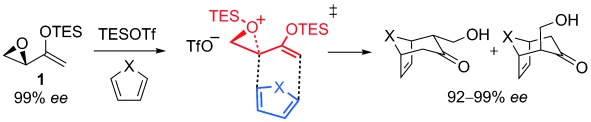
More than one way to add: Theoretical calculations indicate that the silyl-triflate-catalyzed (4+3) cycloadditions of epoxy enolsilane 1 do not generally proceed via an intermediate oxyallyl cation. Instead the reaction involves an SN2-like epoxide ring opening in concert with remote CC bond formation. TES=triethylsilyl, TfO=trifluoromethanesulfonyl.
Perovskite Solar Cells
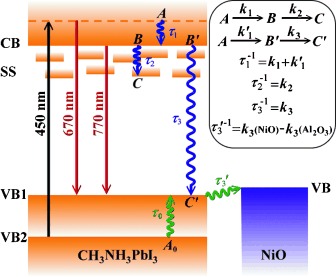
Femtosecond Excitonic Relaxation Dynamics of Perovskite on Mesoporous Films of Al2O3 and NiO Nanoparticles†
- First Published: 02 July 2014
Reaction Mechanisms
Electron Flow in Reaction Mechanisms—Revealed from First Principles†
- First Published: 03 March 2015
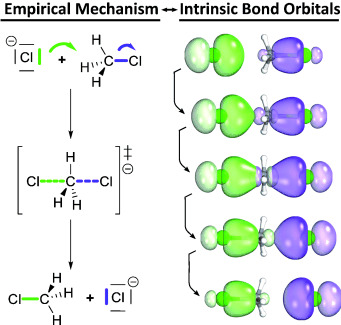
Curly arrows from ab initio calculations: Curly arrows in reaction mechanisms are shown to correspond to changes in intrinsic bond orbitals (IBOs) along reaction paths. With this quantum chemical basis, even complex reaction mechanisms can be derived and visualized in a simple, direct, and intuitive form.
Atmospheric Chemistry
Rapid Autoxidation Forms Highly Oxidized RO2 Radicals in the Atmosphere†
- First Published: 29 October 2014
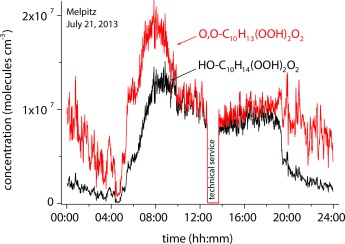
Not only in the solution phase: Highly oxidized RO2 radicals in the atmosphere are rapidly formed by autoxidation initiated by the reaction of O3 and OH radicals with biogenic emissions such as limonene and α-pinene. Field measurements (see picture) confirm experimental findings from a flow-tube study. The closed-shell products from this process represent important aerosol constituents influencing aerosol–cloud–climate interactions.
Surface Chemistry | Very Important Paper
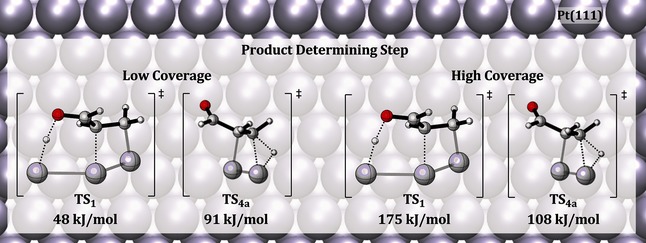
First Principles Calculations for Hydrogenation of Acrolein on Pd and Pt: Chemoselectivity Depends on Steric Effects on the Surface
- First Published: 06 January 2016
Ligand Exchange
Spectra Library: An Assumption-Free In Situ Method to Access the Kinetics of Catechols Binding to Colloidal ZnO Quantum Dots
- First Published: 04 December 2015
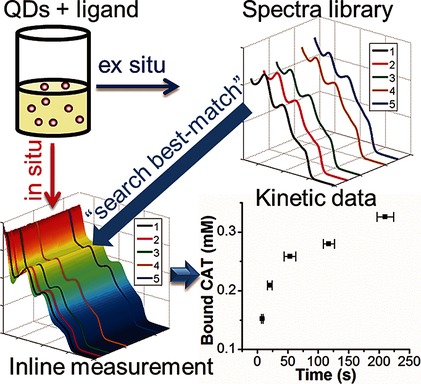
Well-established libraries of target spectra that are derived by means of careful offline analysis and identification of equilibrium data within larger kinetic datasets can be used for any particle–ligand system. Kinetics of ligand binding to nanoparticles can be derived free of assumption, in situ, and with high time resolution.
Kinetic Analysis
A Simple Graphical Method to Determine the Order in Catalyst
- First Published: 08 January 2016

A graphical analysis to elucidate the order in catalyst uses a normalized time scale, t [cat]Tn, to adjust entire reaction profiles constructed with concentration data. Compared to methods that use rates, the normalized time scale analysis requires fewer experiments and minimizes the effects of experimental errors by using information on the entire reaction profile.
Oxygen Electrochemistry
Experimental and Computational Analysis of the Solvent-Dependent O2/Li+-O2− Redox Couple: Standard Potentials, Coupling Strength, and Implications for Lithium–Oxygen Batteries
- First Published: 28 January 2016
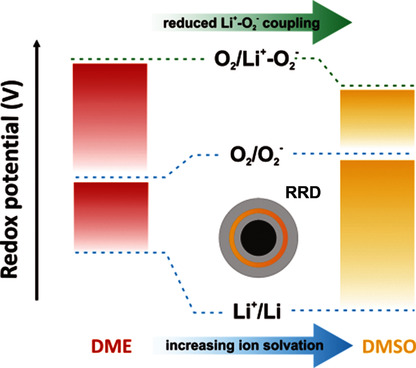
The free-energy landscape of reactions involved in the Li oxygen reduction reaction (ORR) were obtained by rotating ring disk (RRD) measurements and calculations. Differences in redox potentials of O2/O2− and O2/Li+-O2− couples vs. Li+/Li in dimethoxyethane (DME) and dimethylsulfoxide (DMSO) reflect the influence of increasing solvation on the free energy of O2− formation vs. Li+/Li and Li+-O2− coupling.
Polymer Chain Cleavage
Entropy-Driven Selectivity for Chain Scission: Where Macromolecules Cleave
- First Published: 10 December 2015
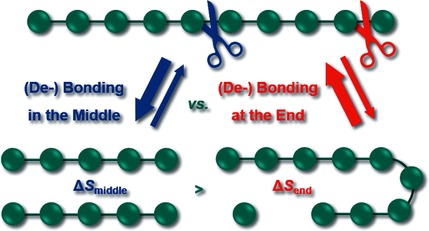
Middle versus end? When all other conditions are equal, bond cleavage in the middle of molecules is entropically much more favored than bond cleavage at the end. Experimental and theoretical approaches were used to study the selectivity of bond cleavage or dissociation of both covalent and supramolecular adducts. The findings have extensive implications for other fields of chemistry.
Surface Chemistry
Isopropanol Dehydration on Amorphous Silica–Alumina: Synergy of Brønsted and Lewis Acidities at Pseudo-Bridging Silanols
- First Published: 02 December 2016
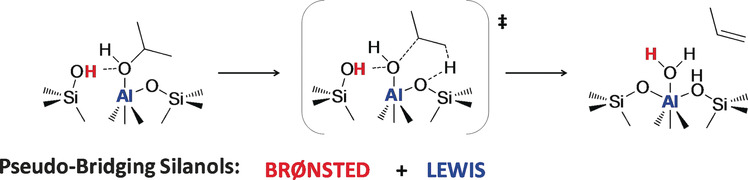
Combining two types of acidity: Periodic ab initio calculations and kinetic measurements were combined to determine the catalytically active sites of isopropanol dehydration on amorphous silica–alumina. Pseudo-bridging silanol sites, which are both Lewis and Brønsted acidic, were found to be of crucial importance in this process.