First Principles Calculations for Hydrogenation of Acrolein on Pd and Pt: Chemoselectivity Depends on Steric Effects on the Surface
Sakari Tuokko
Department of Chemistry, Nanoscience Center, University of Jyväskylä, P.O. Box 35, 40014 Jyväskylä, Finland
Search for more papers by this authorProf. Dr. Petri M. Pihko
Department of Chemistry, Nanoscience Center, University of Jyväskylä, P.O. Box 35, 40014 Jyväskylä, Finland
Search for more papers by this authorCorresponding Author
Dr. Karoliina Honkala
Department of Chemistry, Nanoscience Center, University of Jyväskylä, P.O. Box 35, 40014 Jyväskylä, Finland
Search for more papers by this authorSakari Tuokko
Department of Chemistry, Nanoscience Center, University of Jyväskylä, P.O. Box 35, 40014 Jyväskylä, Finland
Search for more papers by this authorProf. Dr. Petri M. Pihko
Department of Chemistry, Nanoscience Center, University of Jyväskylä, P.O. Box 35, 40014 Jyväskylä, Finland
Search for more papers by this authorCorresponding Author
Dr. Karoliina Honkala
Department of Chemistry, Nanoscience Center, University of Jyväskylä, P.O. Box 35, 40014 Jyväskylä, Finland
Search for more papers by this authorGraphical Abstract
Abstract
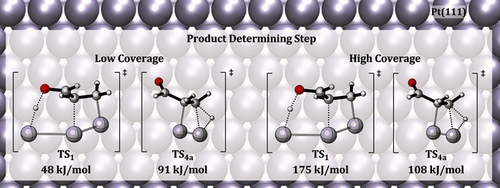
The chemoselective hydrogenation of acrolein on Pt(111) and Pd(111) surfaces is investigated employing density functional theory calculations. The computed potential energy surfaces together with the analysis of reaction mechanisms demonstrate that steric effects are an important factor that governs chemoselectivity. The reactions at the C=O functionality require more space than the reactions at the C=C functionality. Therefore the formation of allyl alcohol is more favorable at low coverage, while the reduction of the C=C bond and the formation of propanal becomes kinetically more favorable at higher coverage. The elementary reaction steps are found to follow different reaction mechanisms, which are identified according to terminology typically used in organometallic catalysis. The transition state scaling (TSS) relationship is demonstrated and the origin of multiple TSS lines is linked to variation of an internal electronic structure of a carbon skeleton.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201507631-sup-0001-misc_information.pdf9.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. Gallezot, D. Richard, Catal. Rev. Sci. Eng. 1998, 40, 81–126;
- 1bP. Mäki-Arvela, J. Hájek, T. Salmi, D. Y. Murzin, Appl. Catal. A 2005, 292, 1–49;
- 1cT. B. L. W. Marinelli, S. Nabuurs, V. Ponec, J. Catal. 1995, 151, 431–438;
- 1dT. B. L. W. Marinelli, V. Ponec, J. Catal. 1995, 156, 51–59;
- 1eV. Ponec, Appl. Catal. A 1997, 149, 27–48;
- 1fP. Beccat, J. C. Bertolini, Y. Gauthier, J. Massardier, P. Ruiz, J. Catal. 1990, 126, 451–456;
- 1gP. Claus, Top. Catal. 1998, 5, 51–62;
- 1hT. Birchem, C.-M. Pradier, Y. Berthier, G. Cordier, J. Catal. 1994, 146, 503–510;
- 1iC. J. Kliewer, M. Bieri, G. A. Somorjai, J. Am. Chem. Soc. 2009, 131, 9958–9966;
- 1jJ. C. de Jesús, F. Zaera, Surf. Sci. 1999, 430, 99–115;
- 1kK. Brandt, M. E. Chiu, D. J. Watson, M. S. Tikhov, R. M. Lambert, J. Am. Chem. Soc. 2009, 131, 17286–17290;
- 1lK. R. Kahsar, D. K. Schwartz, J. W. Medlin, J. Am. Chem. Soc. 2014, 136, 520–526;
- 1mF. Zaera, Chem. Rev. 1995, 95, 2651–2693;
- 1nK.-H. Dostert, C. P. O'Brien, F. Ivars-Barceló, S. Schauermann, H.-J. Freund, J. Am. Chem. Soc. 2015, 137, 13496–13502.
- 2
- 2aJ. W. Medlin, ACS Catal. 2011, 1, 1284–1297;
- 2bD. Loffreda, F. Delbecq, F. Vigné, P. Sautet, Angew. Chem. Int. Ed. 2005, 44, 5279–5282; Angew. Chem. 2005, 117, 5413–5416;
- 2cD. Loffreda, F. Delbecq, F. Vigné, P. Sautet, J. Am. Chem. Soc. 2006, 128, 1316–1323;
- 2dD. Loffreda, F. Delbecq, F. Vigné, P. Sautet, Angew. Chem. Int. Ed. 2009, 48, 8978–8980; Angew. Chem. 2009, 121, 9140–9142;
- 2eS. Laref, F. Delbec, D. Loffreda, J. Catal. 2009, 265, 35–42;
- 2fK. H. Lim, A. B. Mohammad, I. V. Yudanov, K. M. Neyman, M. Bron, P. Claus, N. Rösch, J. Phys. Chem. C 2009, 113, 13231–13240;
- 2gB. Yang, D. Wang, X.-Q. Gong, P. Hu, Phys. Chem. Chem. Phys. 2011, 13, 21146–21152;
- 2hM. S. Ide, B. Hao, M. Neurock, R. J. Davis, ACS Catal. 2012, 2, 671–683;
- 2iD. Loffreda, F. Delbecq, P. Sautet, Chem. Phys. Lett. 2005, 405, 434–439;
- 2jD. Loffreda, Y. Jugnet, F. Delbecq, J. C. Bertolini, P. Sautet, J. Phys. Chem. B 2004, 108, 9085–9093.
- 3
- 3aP. S. Cremer, X. Su, Y. R. Shen, G. A. Somorjai, J. Am. Chem. Soc. 1996, 118, 2942–2949;
- 3bM. Neurock, R. A. van Santen, J. Phys. Chem. B 2000, 104, 11128–11145;
- 3cA. M. H. Rasmussen, M. N. Groves, B. Hammer, ACS Catal. 2014, 4, 1182–1188;
- 3dB. Yang, X.-Q. Gong, H.-F. Wang, X.-M. Cao, J. J. Rooney, P. Hu, J. Am. Chem. Soc. 2013, 135, 15244–15250.
- 4A. D. McNaught, A. Wilkinson in IUPAC Compendium of Chemical Terminology, 2nd ed. (the “Gold Book”), Blackwell Scientific Publications, Oxford, 1997; XML on-line corrected version: http://goldbook.iupac.org (2006-), created by M. Nic, J. Jirat, B. Kosata, updates compiled by A. Jenkins.
- 5
- 5aJ. V. Barth, Surf. Sci. Rep. 2000, 40, 75–149;
- 5bA. U. Nilekar, J. Greeley, M. Mavrikakis, Angew. Chem. Int. Ed. 2006, 45, 7046–7049; Angew. Chem. 2006, 118, 7204–7207.
- 6S. Wang, V. Petzold, V. Tripkovic, J. Kleis, J. G. Howalt, E. Skulason, E. M. Fernandez, B. Hvolbæ, K. G. Jones, A. Toftelund, H. Falsig, M. Bjorketun, F. Studt, F. Abild-Pedersen, J. Rossmeisel, J. K. Nørskov, T. Bligaard, Phys. Chem. Chem. Phys. 2011, 13, 20760–20765.
- 7For details, see the Supporting Information.
- 8
- 8aJ. Enkovaara, et al., J. Phys.: Condens. Matter. 2010, 22, 253202;
- 8bfor more details, see the Supporting Information.
- 9Using the s-cis conformer of acrolein as a reactant leads to the similar results and conclusions as presented in the Supporting Information.
- 10Substrates detected on the surface in the reaction conditions include: carbon monoxide, ethane, propene, ethylidyne and ketene; see Ref. [1i] and [1j].
- 11
- 11aM. Neurock, V. Pallassana, R. A. van Santen, J. Am. Chem. Soc. 2000, 122, 1150–1153;
- 11bF. Calaza, D. Stacchiola, M. Neurock, W. T. Tysoe, J. Am. Chem. Soc. 2010, 132, 2202–2207;
- 11cS. T. Marshall, J. W. Medlin, Surf. Sci. Rep. 2011, 66, 173–184.
- 12Calculated as Δ(TS1−TS4a) for 1/9 ML coverage and Δ(TS1−TS3a) for 1/3 ML coverage).
- 13Calculated as the difference of the highest transition state energies TS12c−TS4b for both coverages.
- 14
- 14aA. S. Loh, S. W. Davis, J. W. Medlin, J. Am. Chem. Soc. 2008, 130, 5507–5514;
- 14bS. T. Marshall, C. M. Horiuchi, W. Zhang, J. W. Medlin, J. Phys. Chem. C 2008, 112, 20406–20412;
- 14cS. H. Pang, J. W. Medlin, J. Phys. Chem. Lett. 2015, 6, 1348–1356.
- 15The calculations were carried out with the RPBE functional but all the main results were also verified with the BEEF-vdW functional. The comparison of these results confirms that van der Waals interactions do not contribute to a reaction mechanism or chemoselectivity.
- 16For a discussion of alternative pathways, see the Supporting Information.
- 17J. F. Hartwig, Organotransition Metal Chemistry: From Bonding to Catalysis, University Science Books, Mill Valley, CA, 2010.
- 18D. R. Weinberg, C. J. Gagliardi, J. F. Hull, C. F. Murphy, C. A. Kent, B. C. Westlake, A. Paul, D. H. Ess, D. G. McCafferty, T. J. Meyer, Chem. Rev. 2012, 112, 4016–4093.
- 19R. I. Masel, Chemical Kinetics and Catalysis. Wiley, New York, 2001, chap. 14, p. 838.
- 20S. G. Wang, B. Temel, J. Shen, G. Jones, L. Grabow, F. Studt, T. Bligaard, F. Abild-Pedersen, C. H. Christensen, J. K. Nørskov, Catal. Lett. 2011, 141, 370–373.
- 21T. R. Munter, T. Bligaard, C. H. Christensen, J. K. Nørskov, Phys. Chem. Chem. Phys. 2008, 10, 5202–5206.
- 22
- 22aE.-U. Würthwein, G. Lang, L. H. Schappele, H. Mayr, J. Am. Chem. Soc. 2002, 124, 4084–4092;
- 22bH. Mayr, A. R. Ofial, Angew. Chem. Int. Ed. 2006, 45, 1844–1854; Angew. Chem. 2006, 118, 1876–1886.