Isopropanol Dehydration on Amorphous Silica–Alumina: Synergy of Brønsted and Lewis Acidities at Pseudo-Bridging Silanols
Corresponding Author
Dr. Kim Larmier
Catalysis and Separation Division, IFP Energies Nouvelles, Echangeur de Solaize, 69360 Solaize, France
Sorbonne Universités, UPMC Univ Paris 06, UMR CNRS 7197, Laboratoire de Réactivité de Surface, Tour 43–33, 3ème étage, Case 178, 4 Place Jussieu, 75252 Paris, France
Search for more papers by this authorCorresponding Author
Dr. Céline Chizallet
Catalysis and Separation Division, IFP Energies Nouvelles, Echangeur de Solaize, 69360 Solaize, France
Search for more papers by this authorDr. Sylvie Maury
Catalysis and Separation Division, IFP Energies Nouvelles, Echangeur de Solaize, 69360 Solaize, France
Search for more papers by this authorDr. Nicolas Cadran
Catalysis and Separation Division, IFP Energies Nouvelles, Echangeur de Solaize, 69360 Solaize, France
Search for more papers by this authorJohnny Abboud
Sorbonne Universités, UPMC Univ Paris 06, UMR CNRS 7197, Laboratoire de Réactivité de Surface, Tour 43–33, 3ème étage, Case 178, 4 Place Jussieu, 75252 Paris, France
Search for more papers by this authorDr. Anne-Félicie Lamic-Humblot
Sorbonne Universités, UPMC Univ Paris 06, UMR CNRS 7197, Laboratoire de Réactivité de Surface, Tour 43–33, 3ème étage, Case 178, 4 Place Jussieu, 75252 Paris, France
Search for more papers by this authorDr. Eric Marceau
Sorbonne Universités, UPMC Univ Paris 06, UMR CNRS 7197, Laboratoire de Réactivité de Surface, Tour 43–33, 3ème étage, Case 178, 4 Place Jussieu, 75252 Paris, France
Search for more papers by this authorProf. Hélène Lauron-Pernot
Sorbonne Universités, UPMC Univ Paris 06, UMR CNRS 7197, Laboratoire de Réactivité de Surface, Tour 43–33, 3ème étage, Case 178, 4 Place Jussieu, 75252 Paris, France
Search for more papers by this authorCorresponding Author
Dr. Kim Larmier
Catalysis and Separation Division, IFP Energies Nouvelles, Echangeur de Solaize, 69360 Solaize, France
Sorbonne Universités, UPMC Univ Paris 06, UMR CNRS 7197, Laboratoire de Réactivité de Surface, Tour 43–33, 3ème étage, Case 178, 4 Place Jussieu, 75252 Paris, France
Search for more papers by this authorCorresponding Author
Dr. Céline Chizallet
Catalysis and Separation Division, IFP Energies Nouvelles, Echangeur de Solaize, 69360 Solaize, France
Search for more papers by this authorDr. Sylvie Maury
Catalysis and Separation Division, IFP Energies Nouvelles, Echangeur de Solaize, 69360 Solaize, France
Search for more papers by this authorDr. Nicolas Cadran
Catalysis and Separation Division, IFP Energies Nouvelles, Echangeur de Solaize, 69360 Solaize, France
Search for more papers by this authorJohnny Abboud
Sorbonne Universités, UPMC Univ Paris 06, UMR CNRS 7197, Laboratoire de Réactivité de Surface, Tour 43–33, 3ème étage, Case 178, 4 Place Jussieu, 75252 Paris, France
Search for more papers by this authorDr. Anne-Félicie Lamic-Humblot
Sorbonne Universités, UPMC Univ Paris 06, UMR CNRS 7197, Laboratoire de Réactivité de Surface, Tour 43–33, 3ème étage, Case 178, 4 Place Jussieu, 75252 Paris, France
Search for more papers by this authorDr. Eric Marceau
Sorbonne Universités, UPMC Univ Paris 06, UMR CNRS 7197, Laboratoire de Réactivité de Surface, Tour 43–33, 3ème étage, Case 178, 4 Place Jussieu, 75252 Paris, France
Search for more papers by this authorProf. Hélène Lauron-Pernot
Sorbonne Universités, UPMC Univ Paris 06, UMR CNRS 7197, Laboratoire de Réactivité de Surface, Tour 43–33, 3ème étage, Case 178, 4 Place Jussieu, 75252 Paris, France
Search for more papers by this authorGraphical Abstract
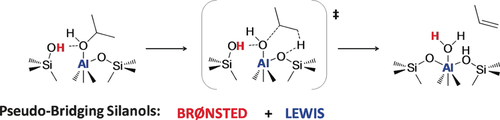
Combining two types of acidity: Periodic ab initio calculations and kinetic measurements were combined to determine the catalytically active sites of isopropanol dehydration on amorphous silica–alumina. Pseudo-bridging silanol sites, which are both Lewis and Brønsted acidic, were found to be of crucial importance in this process.
Abstract
The mechanism of isopropanol dehydration on amorphous silica–alumina (ASA) was unraveled by a combination of experimental kinetic measurements and periodic density functional theory (DFT) calculations. We show that pseudo-bridging silanols (PBS-Al) are the most likely active sites owing to the synergy between the Brønsted and Lewis acidic properties of these sites, which facilitates the activation of alcohol hydroxy groups as leaving groups. Isopropanol dehydration was used to specifically investigate these PBS-Al sites, whose density was estimated to be about 10−1 site nm−2 on the silica-doped alumina surface under investigation, by combining information from experiments and theoretical calculations.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201609494-sup-0001-misc_information.pdf2.2 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G. W. Huber, R. D. Cortright, J. A. Dumesic, Angew. Chem. Int. Ed. 2004, 43, 1549–1551; Angew. Chem. 2004, 116, 1575–1577.
- 2A. Corma, Chem. Rev. 1995, 95, 559–614.
- 3C. P. Bezoukhanova, Y. A. Kalvachev, Catal. Rev. 1994, 36, 125–143.
- 4S. Pega, C. Boissière, D. Grosso, T. Azaïs, A. Chaumonnot, C. Sanchez, Angew. Chem. Int. Ed. 2009, 48, 2784–2787; Angew. Chem. 2009, 121, 2822–2825.
- 5J. F. Stebbins, Z. Xu, Nature 1997, 390, 1996–1998.
- 6G. Busca, Chem. Rev. 2007, 107, 5366–5410.
- 7E. J. M. Hensen, D. G. Poduval, V. Degirmenci, D. A. J. M. Ligthart, W. Chen, M. S. Rigutto, J. A. R. Van Veen, J. Phys. Chem. C 2012, 116, 21416–21429.
- 8S. Sato, M. Toita, T. Sodesawa, F. Nozaki, Appl. Catal. 1990, 62, 73–84.
- 9M. Caillot, A. Chaumonnot, M. Digne, J. A. Van Bokhoven, J. Catal. 2014, 316, 47–56.
- 10G. E. Maciel, J. F. Haw, I. Chuang, B. L. Hawkins, T. A. Early, D. R. Mckay, L. Petrakist, J. Am. Chem. Soc. 1983, 105, 77–83.
- 11N. Y. Topsoe, K. Pedersen, E. G. Derouane, J. Catal. 1981, 70, 41–52.
- 12V. C. Holm, G. C. Bailey, A. Clark, J. Phys. Chem. 1959, 63, 129–133.
- 13G. Crépeau, V. Montouillout, A. Vimont, L. Mariey, T. Cseri, F. Mauge, J. Phys. Chem. B 2006, 110, 15172–15185.
- 14S. Handjani, S. Dzwigaj, J. Blanchard, E. Marceau, J.-M. Krafft, M. Che, Top. Catal. 2009, 52, 334–343.
- 15C. Chizallet, P. Raybaud, ChemPhysChem 2010, 11, 105–108.
- 16C. Chizallet, P. Raybaud, Angew. Chem. Int. Ed. 2009, 48, 2891–2893; Angew. Chem. 2009, 121, 2935–2937.
- 17M. Valla et al., J. Am. Chem. Soc. 2015, 137, 10710–10719.
- 18H. Lauron-Pernot, Catal. Rev. 2006, 48, 315–361.
- 19H. Vinek, J. A. Lercher, H. Noller, J. Mol. Catal. 1985, 30, 353–359.
- 20P. Berteau, B. Delmon, Catal. Today 1989, 5, 121–137.
- 21P. Berteau, M.-A. Kellens, B. Delmon, J. Chem. Soc. Faraday Trans. 1991, 87, 1425.
- 22J. C. Yori, J. C. Luy, J. M. Parera, Appl. Catal. 1988, 41, 1–11.
- 23T. K. Phung, G. Busca, Chem. Eng. J. 2015, 272, 92–101.
- 24T. K. Phung, G. Busca, Catal. Commun. 2015, 68, 110–115.
- 25F. Leydier, C. Chizallet, A. Chaumonnot, M. Digne, E. Soyer, A.-A. Quoineaud, D. Costa, P. Raybaud, J. Catal. 2011, 277, 1–15.
- 26K. Larmier, C. Chizallet, N. Cadran, S. Maury, J. Abboud, A.-F. Lamic-Humblot, E. Marceau, H. Lauron-Pernot, ACS Catal. 2015, 5, 4423–4437.
- 27K. Larmier, A. Nicolle, C. Chizallet, N. Cadran, S. Maury, A.-F. Lamic-Humblot, E. Marceau, H. Lauron-Pernot, ACS Catal. 2016, 6, 1905–1920.
- 28M. John, K. Alexopoulos, M. Reyniers, G. B. Marin, J. Catal. 2015, 330, 28–45.
- 29C. Amatore, A. Jutand, J. Organomet. Chem. 1999, 576, 254–278.
- 30F. A. Carey, R. J. Sundberg, Advanced Organic Chemistry, 5th ed., Springer, USA, 2007.
10.1007/978-0-387-71481-3_2 Google Scholar
- 31F. Leydier, C. Chizallet, D. Costa, P. Raybaud, J. Catal. 2015, 325, 35–47.
- 32X. Rozanska, R. van Santen, F. Hutschka, J. Hafner, J. Am. Chem. Soc. 2001, 123, 7655–7667.
- 33S. S. M. Konda, S. Caratzoulas, D. G. Vlachos, ACS Catal. 2016, 6, 123–133.
- 34J. P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 1996, 77, 3865–3868.
- 35G. Kresse, J. Hafner, Phys. Rev. B 1994, 49, 14251–14269.
- 36G. Kresse, J. Furthmüller, Phys. Rev. B 1996, 54, 11169–11186.
- 37G. Kresse, D. Joubert, Phys. Rev. B 1999, 59, 1758–1775.
- 38S. Grimme, J. Comput. Chem. 2006, 27, 1787–1799.
- 39G. Henkelman, B. P. Uberuaga, H. Jónsson, J. Chem. Phys. 2000, 113, 9901–9904.
- 40G. Henkelman, H. Jónsson, J. Chem. Phys. 2000, 113, 9978–9985.