Rationalization of an Unusual Solvent-Induced Inversion of Enantiomeric Excess in Organocatalytic Selenylation of Aldehydes†
A.A. and D.G.B. acknowledge funding from the EPSRC. D.G.B. acknowledges funding from the NSF (CHE-1123895). J.B. acknowledges a postdoctoral fellowship from the Education Ministry of Spain (EX2009-0687) and FECYT and a Junior Research Fellowship from Imperial College. We acknowledge D.-H. Huang and L. Pasternack (TSRI NMR Facility) for valuable NMR assistance and Dr. M. Roggen and J. Wiest for preliminary experiments.
Graphical Abstract
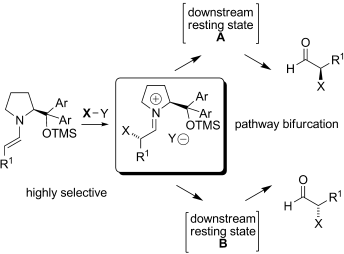
Off the beaten path: Studies of an unusual inversion of the sense of enantioselectivity for the selenylation of aldehydes catalyzed by a diphenylprolinol ether catalyst provides support for a mechanistic concept beyond the simple steric model developed for enamine catalysis in these systems. A general role for downstream intermediates in selectivity outcomes in organocatalysis is discussed. TMS=trimethylsilyl.
Abstract
An unusual solvent-induced inversion of the sense of enantioselectivity observed in the α-selenylation of aldehydes catalyzed by a diphenylprolinol silyl ether catalyst is correlated to the presence of intermediates formed subsequent to the highly selective CSe bond-forming step in the catalytic cycle. This work provides support for a mechanistic concept for enamine catalysis and includes a general role for “downstream intermediates” in selectivity outcomes in organocatalysis.