Journal list menu
Export Citations
Download PDFs
Cover Pictures
Cover Picture: Catalytic Processes that Changed the World: 100 Years Max-Planck-Institut für Kohlenforschung (Angew. Chem. Int. Ed. 33/2014)
- Page: 8525
- First Published: 30 July 2014

Some of the most important chemical discoveries of the last 100 years were made at the Max-Planck-Institut für Kohlenforschung (MPI) in Mülheim an der Ruhr, which was founded in 1914—100 years ago—as the Kaiser-Wilhelm-Institut für Kohlenforschung. A look at history illustrates the scientific potential of the Mülheim basic research: In 1925, F. Fischer and H. Tropsch filed a patent for the Fischer–Tropsch synthesis—gasoline from coal. The low-pressure approach to polyethylene was patented in 1953 by K. Ziegler, H. Breil, E. Holzkamp, and H. Martin. Ziegler was awarded the Nobel Prize in 1963 for this work. The decaffeination of coffee by supercritical carbon dioxide was patented in 1970 by K. Zosel. This Issue, starting with an Editorial by B. List on page 8528 ff., contains contributions from researchers, who have had a close connection in one way or another with this extraordinary Institution during their scientific careers. A summary of the history of the “KoFo-MPI” is given in the Essay by M. Reetz on page 8562 ff.
Inside Cover: Catalytic Biorefining of Plant Biomass to Non-Pyrolytic Lignin Bio-Oil and Carbohydrates through Hydrogen Transfer Reactions (Angew. Chem. Int. Ed. 33/2014)
- Page: 8526
- First Published: 07 August 2014

Lignin solvolytically released from biomass may be of much lower molecular weights than currently believed. In their Communication on page 8634 ff., R. Rinaldi and P. Ferrini exploit this feature for establishing a method that enables the isolation of lignin as a depolymerized oil and yields pulps amenable to enzymatic hydrolysis. The lignin oil is highly susceptible to hydrogenation under mild conditions, and therefore, a unique pathway for lignin valorization by heterogeneous catalysis has been established.
Inside Back Cover: A Brønsted Acid Catalyzed Redox Arylation (Angew. Chem. Int. Ed. 33/2014)
- Page: 8789
- First Published: 27 June 2014

The catalytic redox-neutral arylation of ynamides with aryl sulfoxides is described by N. Maulide et al. in their Communication on page 8718 ff. This reaction is illustrated by the harmonious catalysis of a proton that joins the two reagents in an atom-economic manner. The background depicts the harmonious collaboration between the two institutions where this work was carried out: the Max-Planck-Institut für Kohlenforschung and the University of Vienna.
Back Cover: (Angew. Chem. Int. Ed. 33/2014)
- Page: 8790
- First Published: 30 July 2014

Five departments, one common goal:For the past 100 years, the Max-Planck-Institut für Kohlenforschung in Mülheim an der Ruhr has focused above all on chemical catalysis, and this is more true today than ever. Researchers in heterogeneous, homogeneous, and biocatalysis, organometallic chemistry, organic synthesis, as well as theoretical chemistry are occupied with the catalytic transformation of raw materials and pure compounds with the highest possible chemo-, regio-, and stereoselectivity under conditions that maximize the use of natural resources.
Frontispiece
Frontispiece: The Virtue of Defects: Stable Bromine Production by Catalytic Oxidation of Hydrogen Bromide on Titanium Oxide
- First Published: 07 August 2014

N. López, J. Pérez-Ramírez, and co-workers show on page 8628 ff. that HBr doping of the rutile TiO2 surface generates impurity levels with electrons that can be injected into or recovered from the reactants at the right energies, transforming the catalytically inert semiconductor into an active catalyst.
Editorial
Catalytic Processes that Changed the World: 100 Years Max-Planck-Institut für Kohlenforschung
- Pages: 8528-8530
- First Published: 27 July 2014

“…︁ In this special issue of Angewandte Chemie, which is indeed very special for us, you as a reader should get an impression of what, in our opinion, constitutes the research in and around the Max-Planck-Institut für Kohlenforschung. You will notice the diversity of the research topics that are covered by researchers at the Institute …︁ Read more in the Editorial by Benjamin List.
Graphical Abstract
Graphical Abstract: Angew. Chem. Int. Ed. 33/2014
- Pages: 8532-8544
- First Published: 07 August 2014
News
Spotlights on our sister journals: Angew. Chem. Int. Ed. 33/2014
- Pages: 8546-8549
- First Published: 07 August 2014
Author Profile
News
Vice-President of the Max Planck Society: F. Schüth / Robert Bunsen Lectureship: W. Thiel / Honorary Membership of the Gesellschaft Deutscher Chemiker: G. Wilke / Chirality Award: M. T. Reetz / Prince of Asturias Award: A. Corma, M. E. Davis, and G. D. Stucky / Pauling Medal: S. L. Buchwald / August Wilhelm von Hofmann Memorial Medal: B. M. Trost / Emil Fischer Medal: M. Beller
- Pages: 8552-8553
- First Published: 23 July 2014
Highlights
Heterogeneous Catalysis
Breakthroughs in Hard X-ray Diffraction: Towards a Multiscale Science Approach in Heterogeneous Catalysis†
- Pages: 8556-8558
- First Published: 02 July 2014
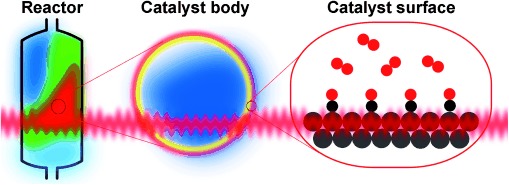
Diffraction at hard work: Modern heterogeneous catalysis would benefit from a multiscale science approach bridging the molecular world with the macroscopic world. Because of recent breakthroughs in X-ray diffraction methods, including the surface X-ray diffraction of atomically flat model catalysts, X-ray diffraction tomography of catalyst bodies, and X-ray profiling of an active catalyst in a chemical reactor, such an approach is now within reach.
Renewable Resources
Plant Biomass Fractionation Meets Catalysis†
- Pages: 8559-8560
- First Published: 15 July 2014

Catalytic biorefining: Beyond the deconstruction of plant biomass, emerging processes for catalytic biomass fractionation are directed towards rationally designing the properties of the isolated biomass components through and for catalysis. The full potential of catalysis in the conversion of the isolated fractions into chemicals and fuels can thus be exploited.
Essays
100 Years of Catalysis
One Hundred Years of the Max-Planck-Institut für Kohlenforschung
- Pages: 8562-8586
- First Published: 24 June 2014

Catalysis pure: This Essay is an account of the institutional and scientific development of the Max-Planck-Institut für Kohlenforschung in Mülheim an der Ruhr (Germany), which is the successor to the Kaiser-Wilhelm-Institut für Kohlenforschung founded in 1914. It is an institute that has focused on catalysis for 100 years. Key historical events, organizational changes, and research highlights of four major periods are featured.
Catalysis in Mülheim
Catalysis for Total Synthesis: A Personal Account
- Pages: 8587-8598
- First Published: 01 July 2014

Divining rod: Natural product synthesis often serves as a divining rod in our search for new and useful catalytic reactivity. This personal account—written in honor of the Max-Planck-Institut für Kohlenforschung on the occasion of its 100th anniversary—summarizes the major lines of research pursued in the author's laboratory, which show how total synthesis and research into basic organometallic chemistry cross-fertilize each other.
Heterogeneous Catalysis
Control of Solid Catalysts Down to the Atomic Scale: Where is the Limit?
- Pages: 8599-8604
- First Published: 07 July 2014
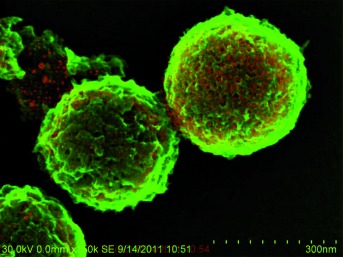
Down to the last detail: Nanostructured solid catalysts were already known in the early 20th century, but their exact structure was unclear. Nowadays, the arrangement of atoms and particles in solids can be manipulated and analyzed down to the atomic scale (see image). The use of specific highly active catalysts enables industrially relevant reactions to be performed at room temperature.
Computational Chemistry
Electron Microscopy
Heterogeneous Catalysis
Catalytic Paradigms: A Riddle and a Puzzle
- Pages: 8618-8620
- First Published: 02 June 2014
The perfect catalyst: The advances towards the ability to design a catalyst from first principles are explored. Aspects of computational chemistry as well as the kinetics and physical state of the reactive catalyst are discussed.
Zeolites in Biorefineries
Will Zeolite-Based Catalysis be as Relevant in Future Biorefineries as in Crude Oil Refineries?
- Pages: 8621-8626
- First Published: 25 July 2014
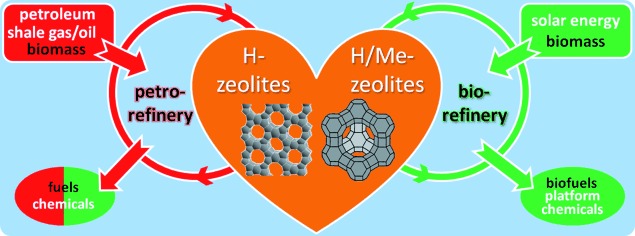
Approved catalysts for fuels of the future: Transition from petroleum- to biomass-based fuel economy will require new conversion strategies. This Essay describes how recent developments in zeolite synthesis and modification allow adapting zeolite properties to achieve selective conversion of biomass compounds.
Communications
Defects in Catalysis
The Virtue of Defects: Stable Bromine Production by Catalytic Oxidation of Hydrogen Bromide on Titanium Oxide
- Pages: 8628-8633
- First Published: 02 June 2014
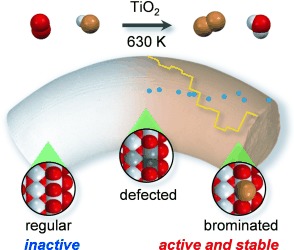
Who wants to be perfect? Rutile TiO2 has a limited use in heterogeneous catalysis owing to its inertness and its relatively low surface area. The in situ generation of defect states on the rutile surface enables the dissociation of molecular oxygen, transforming this semiconductor into a highly active, stable, and cost-effective catalyst. It catalyzes the oxidation of HBr to Br2, an important reaction for halogen-mediated alkane functionalization processes.
Heterogeneous Catalysis
Catalytic Biorefining of Plant Biomass to Non-Pyrolytic Lignin Bio-Oil and Carbohydrates through Hydrogen Transfer Reactions†
- Pages: 8634-8639
- First Published: 11 June 2014
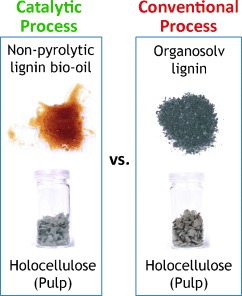
Lignin finds its true destiny: A catalytic biorefining method results in the isolation of depolymerized lignin, that is, a non-pyrolytic lignin bio-oil, in addition to pulps that are amenable to enzymatic hydrolysis. The lignin bio-oil is highly susceptible to hydrogenation under mild conditions, and therefore, a unique pathway for lignin valorization by heterogeneous catalysis has been established.
Asymmetric Catalysis
Monitoring Surface Processes During Heterogeneous Asymmetric Hydrogenation of Ketones on a Chirally Modified Platinum Catalyst by Operando Spectroscopy†
- Pages: 8640-8644
- First Published: 28 April 2014
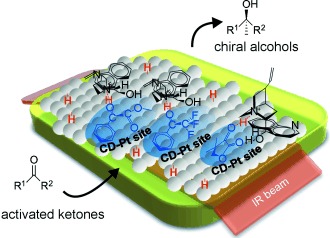
Watching the working catalyst: Surface processes occurring at the catalytic chiral surface of a cinchona-modified Pt catalyst during the asymmetric hydrogenation of activated ketones have been monitored by attenuated total reflection IR spectroscopy (see picture). Fundamental information about this catalytic system could be gained.
Polymeric Catalysts | Hot Paper
A Polyphenylene Support for Pd Catalysts with Exceptional Catalytic Activity†
- Pages: 8645-8648
- First Published: 15 July 2014
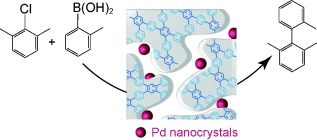
Support with benefits: A composite catalyst is synthesized by palladium-catalyzed Suzuki coupling which directly results in formation of palladium nanoparticles confined to a porous polyphenylene network. A polyphenylene support serves as an excellent platform for metal-catalyzed reactions, which are typically carried out under homogeneous conditions.
GFP Photochemistry
Concerted Asynchronous Hula-Twist Photoisomerization in the S65T/H148D Mutant of Green Fluorescent Protein†
- Pages: 8649-8653
- First Published: 14 July 2014
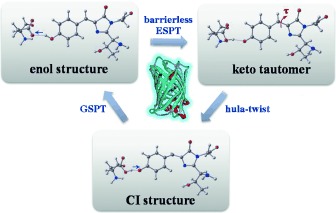
GFP mutant likes the hula-twist: QM/MM calculations show that the protein environment makes the S65T/H148D green fluorescent protein (GFP) double mutant adopt the concerted asynchronous hula-twisting pathway for isomerization (see picture), instead of the one-bond flip favored in its absence. Hydrogen out-of-plane motion is for the first time found to play an important role in GFP deactivation.
Synthetic Methods
Rapid Access to β-Trifluoromethyl-Substituted Ketones: Harnessing Inductive Effects in Wacker-Type Oxidations of Internal Alkenes†
- Pages: 8654-8658
- First Published: 18 July 2014
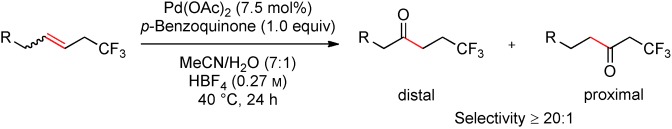
Synthetically highly desirable β-trifluoromethyl-substituted ketones can be rapidly accessed by a trifluoromethyl-directed Wacker oxidation starting from alkenes bearing an allylic trifluoromethyl group (see scheme). This effect seems to be dominantly inductive and can override coordinative effects. The reaction has a broad substrate scope and affords products in high yields and very high regioselectivity.
Biocatalysis
Cytochrome P450 Catalyzed Oxidative Hydroxylation of Achiral Organic Compounds with Simultaneous Creation of Two Chirality Centers in a Single CH Activation Step†
- Pages: 8659-8663
- First Published: 03 March 2014
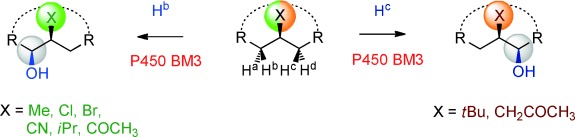
Two birds with one stone: Two new centers of chirality are created by the cytochrome P450 catalyzed regio-, diastereo-, and enantioselective oxidative hydroxylation of appropriate achiral compounds. When using either wild type cytochrome P450 BM3 or mutants generated by directed evolution, a high preference for one of the four stereotopic H atoms is observed depending on the nature of the X moiety in the substrate.
Molecular Dynamics
Competition Between Concerted and Stepwise Dynamics in the Triplet Di-π-Methane Rearrangement†
- Pages: 8664-8667
- First Published: 11 March 2014
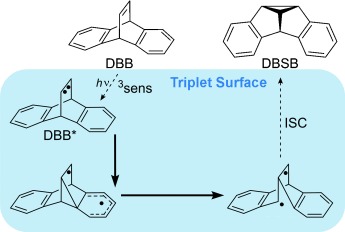
Once in a lifetime: The molecular dynamics of the triplet-state Zimmerman di-π-methane rearrangement of dibenzobarrelene (DBB) were computed. All productive quasiclassical trajectories involve sequential formation and cleavage of CC bonds and an intermediate with lifetimes ranging from 13 to 1160 fs. Such short lifetimes indicate the nonstatistical nature of the reaction mechanism. DBSB=dibenzosemibullvalene, ISC=intersystem crossing.
Asymmetric Hydrogenation
Asymmetric Hydrogenation of α,β-Unsaturated Nitriles with Base-Activated Iridium N,P Ligand Complexes†
- Pages: 8668-8671
- First Published: 20 March 2014
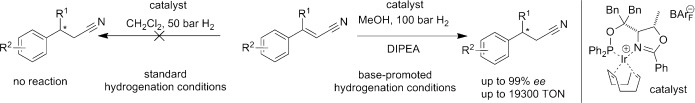
The base makes the difference. Addition of N,N-diisopropylethylamine (DIPEA) or the presence of a basic counterion dramatically enhance the activity of iridium catalysts in the asymmetric hydrogenation of α,β-unsaturated nitriles, a substrate class for which no suitable catalysts were available before. Under these conditions a cyano-substituted CC bond can be selectively reduced, leaving less electrophilic CC bonds intact.
Theory of Catalysis
Computational Kinetics of Cobalt-Catalyzed Alkene Hydroformylation†
- Pages: 8672-8676
- First Published: 03 April 2014
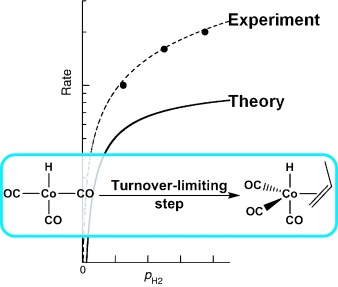
Cobalt bottlenecks: Density functional theory, coupled-cluster theory, and transition state theory are used to build a computational model of the kinetics of phosphine-free cobalt-catalyzed hydroformylation and hydrogenation of alkenes. The model provides very good agreement with experiment (see picture), and enables the factors that determine the selectivity and rate of catalysis to be established. The turnover rate is mainly determined by the alkene coordination step.
CH Activation
Copper-Catalyzed Regioselective ortho CH Cyanation of Vinylarenes†
- Pages: 8677-8681
- First Published: 06 May 2014
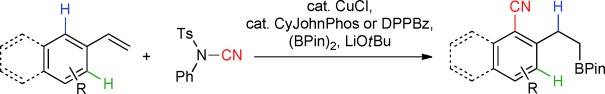
Reaction combo: A combined copper-catalyzed hydroborylation/ortho CH cyanation of vinylarenes is described, thus allowing the selective functionalization of vinylarenes and featuring unique site selectivity. The reaction leads to ortho-selective CH functionalization of arenes and anti-Markovnikov hydrofunctionalization of the pendant olefin (see scheme; Pin=pinacolato, Ts=4-toluenesulfonyl).
Photochemistry
Photocaging of Carboxylic Acids: A Modular Approach†
- Pages: 8682-8686
- First Published: 06 May 2014
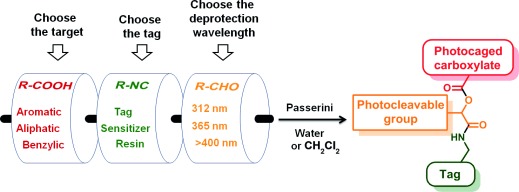
Mix and match: The multicomponent Passerini reaction is used for the preparation of photocaged carboxylic acids, both in dichloromethane and water. Judicious choice of the aldehyde allows tuning of the deprotection wavelength and the preparation of orthogonally protected products. The isocyanide component may be used for immobilization on a solid support or introduction of either a reactive tag or a photosensitizer.
Heterogeneous Domino Catalysis
Multisite Organic–Inorganic Hybrid Catalysts for the Direct Sustainable Synthesis of GABAergic Drugs†
- Pages: 8687-8690
- First Published: 18 June 2014
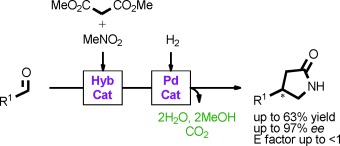
Seven steps in two pots: Organic–inorganic hybrid catalysts have been prepared and applied in a new general, practical, and flexible synthetic procedure toward industrially relevant GABA derivatives. The domino sequence is composed of seven chemical transformations which are performed in two one-pot reactions. The method produces both enantiomeric forms of the product in high enantiopurity as well as the racemate in good yields.
Asymmetric Catalysis
Iridium-Catalyzed Enantioselective Allylic Substitution of Unstabilized Enolates Derived from α,β-Unsaturated Ketones†
- Pages: 8691-8695
- First Published: 10 June 2014
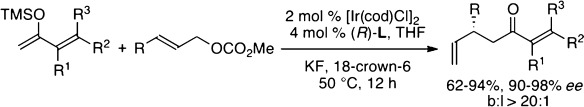
Highly selective: An Ir-catalyzed enantioselective allylic substitution reaction of unstabilized silyl enolates derived from α,β-unsaturated ketones was developed. Reaction with a variety of allylic carbonates gave allylated products in good yields with high enantioselectivities and excellent branched-to-linear selectivities. The synthetic utility was demonstrated in the synthesis of an anticancer agent, TEI-9826.
Homogeneous Catalysis
Palladium-Catalyzed Oxidative Arylating Carbocyclization of Allenynes: Control of Selectivity and Role of H2O†
- Pages: 8696-8699
- First Published: 30 June 2014
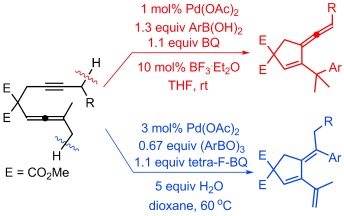
Selective protocols are described for the carbocyclization/arylation of allenynes using arylboronic acids that allow for the formation of either arylated vinylallenes or arylated trienes. Formation of the former product is promoted by the use of BF3⋅Et2O whereas the latter product is selectively formed when water is used as an additive. Water plays a crucial role for the product distribution.
Organocatalysis
Rationalization of an Unusual Solvent-Induced Inversion of Enantiomeric Excess in Organocatalytic Selenylation of Aldehydes†
- Pages: 8700-8704
- First Published: 07 July 2014
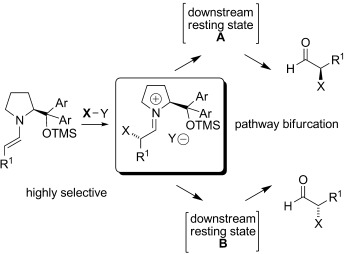
Off the beaten path: Studies of an unusual inversion of the sense of enantioselectivity for the selenylation of aldehydes catalyzed by a diphenylprolinol ether catalyst provides support for a mechanistic concept beyond the simple steric model developed for enamine catalysis in these systems. A general role for downstream intermediates in selectivity outcomes in organocatalysis is discussed. TMS=trimethylsilyl.
Molecular Diversity
Efficient and Modular Synthesis of New Structurally Diverse Functionalized [n]Paracyclophanes by a Ring-Distortion Strategy†
- Pages: 8705-8708
- First Published: 30 June 2014
![Efficient and Modular Synthesis of New Structurally Diverse Functionalized [n]Paracyclophanes by a Ring-Distortion Strategy](/cms/asset/9ce0da90-af9b-4d58-9d4b-ac22b2e4cd75/mcontent.jpg)
An efficient, modular, and simple access to new diverse functionalized [n]paracyclophanes, incorporating heteroatoms and structural features such as aryl ethers, biaryl subunits, or lactams, as well as original cage architectures, has been developed from readily available building blocks. The approach relies on sequential Diels–Alder/retro-Diels–Alder reactions.
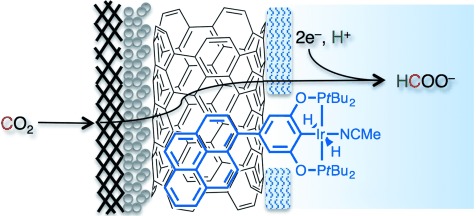
CO2 Reduction
Rapid Selective Electrocatalytic Reduction of Carbon Dioxide to Formate by an Iridium Pincer Catalyst Immobilized on Carbon Nanotube Electrodes†
- Pages: 8709-8713
- First Published: 04 June 2014
Organocatalysis
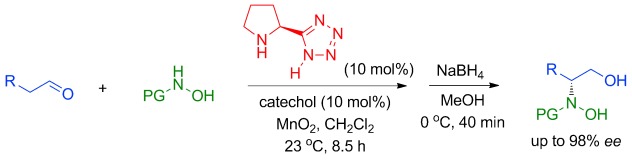
Proline-Tetrazole-Catalyzed Enantioselective N-Nitroso Aldol Reaction of Aldehydes with In Situ Generated Nitrosocarbonyl Compounds†
- Pages: 8714-8717
- First Published: 19 February 2014
Redox Catalysis | Hot Paper
A Brønsted Acid Catalyzed Redox Arylation†
- Pages: 8718-8721
- First Published: 03 March 2014
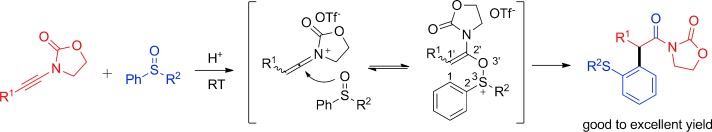
Playing a neutral game: A Brønsted acid catalyzed redox-neutral arylation of ynamides with aryl sulfoxides is described. A broad range of ynamides were converted into the corresponding α-arylated oxazolidinones in good to excellent yields under mild conditions and in an atom-economic fashion. Tf=trifluoromethylsulfonyl.
Iron Catalysis
Hydrogenation of Esters to Alcohols with a Well-Defined Iron Complex
- Pages: 8722-8726
- First Published: 30 May 2014
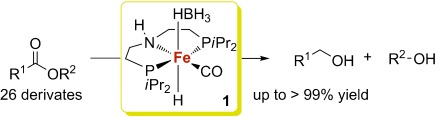
A brilliant progressIRON: A novel and efficient catalyst system based on iron complex 1 has been developed for the hydrogenation of various aromatic and aliphatic carboxylic acid esters as well as lactones towards the corresponding alcohols. The reaction is postulated to proceed by an outer-sphere mechanism.
Bioinorganic Chemistry | Very Important Paper
Decay of Iron(V) Nitride Complexes By a NN Bond-Coupling Reaction in Solution: A Combined Spectroscopic and Theoretical Analysis†
- Pages: 8727-8731
- First Published: 18 May 2014
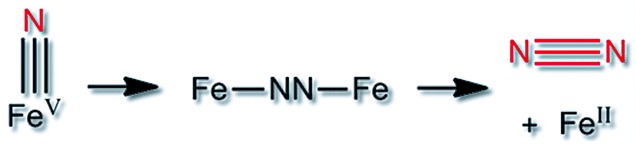
Always look on the bright azide of life: the qualitative formation of dinitrogen results from the decay of FeV nitride complexes and is investigated by a combination of spectroscopy, spectrometry, and theory. New insight is obtained in the photolysis of iron azide complexes, which is crucial for the formation of iron nitrides.
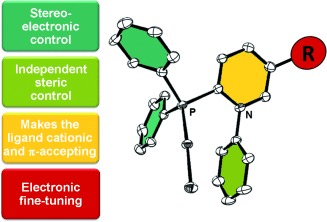
Cationic Phosphines | Hot Paper
Synthesis, Structure, and Applications of Pyridiniophosphines†
- Pages: 8732-8736
- First Published: 07 May 2014
Synthetic Methods
Acid-Catalyzed Oxidative Radical Addition of Ketones to Olefins†
- Pages: 8737-8740
- First Published: 28 April 2014
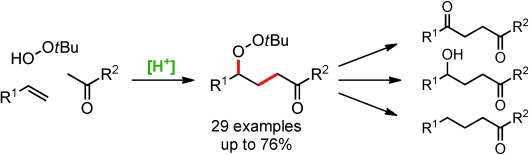
A radical combination: A multicomponent radical addition of unactivated ketones and hydroperoxide to styrenes under Brønsted acid catalysis provides a range of γ-peroxyketones. The products can be further transformed into 1,4-diketones, homoaldol compounds, and alkyl ketones. The formation of radicals is believed to proceed via thermally labile alkenylperoxides, which are generated in situ.
NHC Complexes
Bis-NHC Chelate Complexes of Nickel(0) and Platinum(0)†
- Pages: 8741-8745
- First Published: 21 May 2014
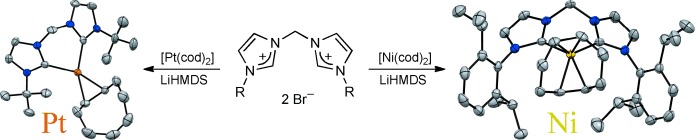
Adjusting the bite: d10-ML2 fragments are well known to activate unreactive bonds by oxidative addition. Starting from lithium carbene adducts formed in situ, nickel and platinum olefin complexes of such fragments bearing chelating bis-NHCs were synthesized. Combining these strong electron donors with the bent coordination geometry enforced by chelation opens interesting perspectives for bond-activation chemistry and catalysis.
BF3-Mediated Coupling
Transition-Metal-Free BF3-Mediated Oxidative and Non-Oxidative Cross-Coupling of Pyridines†
- Pages: 8746-8750
- First Published: 22 April 2014
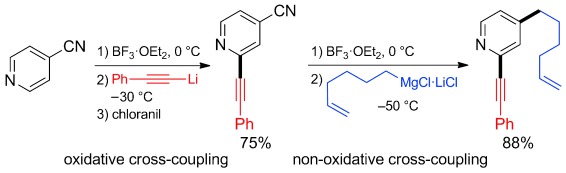
Oxidative or non-oxidative—That is the question! Pyridines bearing a substituent at position 4 readily undergo a BF3-mediated oxidative coupling at position 2 with a wide range of alkynyllithium compounds. In contrast, 4-cyano- or 4-chloropyridines undergo a novel BF3-mediated cross-coupling at position 4 with alkylmagnesium reagents. The combination of the two transition-metal-free procedures allows the preparation of a broad range of pyridines.
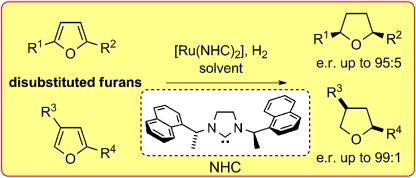
Asymmetric Hydrogenation
Asymmetric Hydrogenation of Disubstituted Furans†
- Pages: 8751-8755
- First Published: 19 February 2014
Tropos Organocatalyst
Selector-Induced Dynamic Deracemization of a Selectand-Modified Tropos BIPHEPO-Ligand: Application in the Organocatalyzed Asymmetric Double-Aldol-Reaction†
- Pages: 8756-8760
- First Published: 25 June 2014
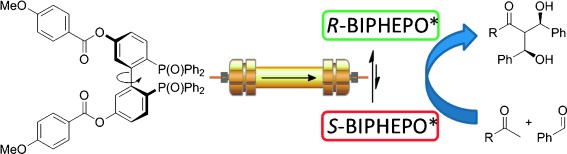
Deracemization does the trick: The catalytic chiral resolution of tropos biarylphosphineoxides is combined with their high stereoinduction in asymmetric double-aldol-additions. The transient diastereomeric interaction of the modified biarylphosphineoxide was maximized by molecular design, which leads to an unusually high deracemization during HPLC.
Colloidal Crystals
Observation of Nano-Dewetting in Colloidal Crystal Drying†
- Pages: 8761-8764
- First Published: 22 May 2014
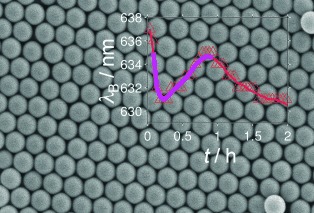
Short breath: The continuous shrinkage of a colloidal crystal during drying is spontaneously interrupted several minutes after starting the drying. The system seems to take a breath before it shrinks monotonously until its final state after about one day. This short period becomes visible by a short red-shift of the Bragg peak characterizing the lattice constant.
Organocatalysis
Towards High-Performance Lewis Acid Organocatalysis†
- Pages: 8765-8769
- First Published: 06 May 2014
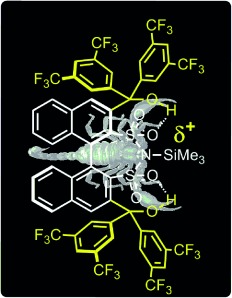
Ready to attack! The combination of Lewis acid organocatalysis and internal hydrogen-bond assistance was used to develop a new type of highly active disulfonimide catalyst. The increased Lewis acidity was documented by activity comparisons and theoretical investigations. The potential of the hydrogen-bond-assisted disulfonimide catalyst was demonstrated by its application in an enantioselective transformation.
Organocatalyis
Catalytic Asymmetric Torgov Cyclization: A Concise Total Synthesis of (+)-Estrone†
- Pages: 8770-8773
- First Published: 30 July 2014
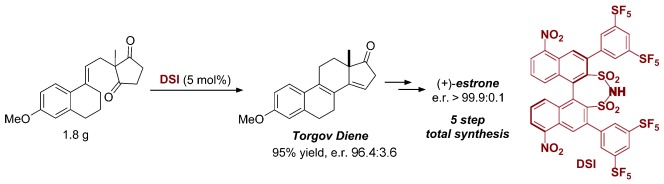
A short circuit: An asymmetric Torgov cyclization, catalyzed by a novel, highly Brønsted acidic dinitro-substituted disulfonimide, is described. The reaction delivers the Torgov diene and various analogues with excellent yields and enantioselectivity. The method was applied in a very short synthesis of (+)-estrone.
CH Activation on MgO
Sites for Methane Activation on Lithium-Doped Magnesium Oxide Surfaces†
- Pages: 8774-8778
- First Published: 23 April 2014
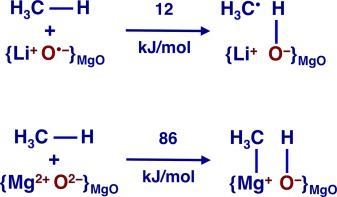
The energy barriers for methane activation by hydrogen abstraction at oxygen radical sites are unrealistically low. CH bonds can also heterolytically add onto a regular MgO ion pair at morphological defects such as steps and corners. Release of methyl radicals into the gas phase will happen only in the presence of O2.
β-Amino Acids
Stereoselective Metal-Free Synthesis of β-Amino Thioesters with Tertiary and Quaternary Stereogenic Centers†
- Pages: 8779-8783
- First Published: 18 March 2014
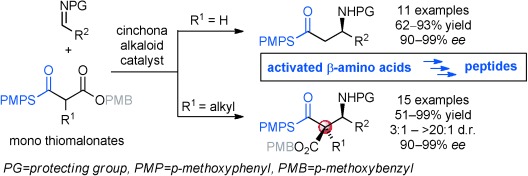
Thioesters on the rise: β-Amino thioesters with tertiary and even quaternary stereogenic centers can be formed with high diastereo- and enantioselectivities from Mannich reactions with monothiomalonates in the presence of catalytic amounts of cinchona alkaloids. The synthetic value of the β-amino thioesters as preactivated β-amino acids was, for example, shown in coupling-reagent-free peptide synthesis.
Remote-Controlled Reactivity
Controlling Covalent Connection and Disconnection with Light†
- Pages: 8784-8787
- First Published: 24 February 2014
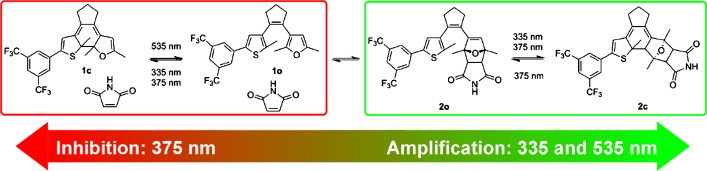
Remote-controlled equilibrium: Gating both sides of a reversible covalent Diels–Alder reaction by a photoswitch allows control over the connection and disconnection of two chemical entities and shifting of their equilibrium by light. This approach should prove particularly powerful when designing dynamic cross-linked polymers and for reversible covalent functionalization of sp2-hybridized carbon allotropes, such as graphene and carbon nanotubes.