Asymmetric Hydrogenation of Disubstituted Furans†
Jędrzej Wysocki
NRW Graduate School of Chemistry, Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/glorius/
Search for more papers by this authorDr. Nuria Ortega
Bayer Pharma AG, Medicinal Chemistry, Aprather Weg 18 A, 42113 Wuppertal (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Frank Glorius
NRW Graduate School of Chemistry, Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/glorius/
NRW Graduate School of Chemistry, Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/glorius/Search for more papers by this authorJędrzej Wysocki
NRW Graduate School of Chemistry, Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/glorius/
Search for more papers by this authorDr. Nuria Ortega
Bayer Pharma AG, Medicinal Chemistry, Aprather Weg 18 A, 42113 Wuppertal (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Frank Glorius
NRW Graduate School of Chemistry, Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/glorius/
NRW Graduate School of Chemistry, Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstrasse 40, 48149 Münster (Germany) http://www.uni-muenster.de/Chemie.oc/glorius/Search for more papers by this authorWe thank Dr. Matthew N. Hopkinson for helpful discussions during preparation of this manuscript and Prof. Dr. Klaus Ditrich (BASF) for donation of precious chiral amines (ChiPros). This work was supported by the NRW Graduate School of Chemistry in Münster (J.W.).
Graphical Abstract
Abstract
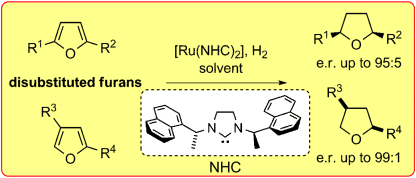
An enantioselective hydrogenation of disubstituted furans has been developed by using a chiral ruthenium catalyst with N-heterocyclic carbene ligands. This reaction converts furans into valuable enantioenriched disubstituted tetrahydrofurans.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201310985_sm_miscellaneous_information.pdf7.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on the hydrogenation of aromatic compounds, see
- 1aP. J. Dyson, Dalton Trans. 2003, 2964;
- 1bF. Glorius, Org. Biomol. Chem. 2005, 3, 4171;
- 1cY.-G. Zhou, Acc. Chem. Res. 2007, 40, 1357;
- 1dR. Kuwano, Heterocycles 2008, 76, 909;
- 1eD.-S. Wang, Q.-A. Chen, S.-M. Lu, Y.-G. Zhou, Chem. Rev. 2012, 112, 2557;
- 1fZ. Yu, W. Jin, Q. Jiang, Angew. Chem. 2012, 124, 6164; Angew. Chem. Int. Ed. 2012, 51, 6060;
- 1gD. Zhao, F. Glorius, Angew. Chem. 2013, 125, 9794; Angew. Chem. Int. Ed. 2013, 52, 9616.
- 2
- 2aJ. P. Wolfe, M. B. Hay, Tetrahedron 2007, 63, 261;
- 2bJ.-Y. Pan, S.-L. Chen, M.-H. Yang, J. Wu, J. Sinkkonen, K. Zou, Nat. Prod. Rep. 2009, 26, 1251;
- 2cA. Lorente, J. Lamariano-Merketegi, F. Albericio, M. Álvarez, Chem. Rev. 2013, 113, 4567.
- 3For selected examples of the asymmetric hydrogenation of quinolines, see
- 3aW.-B. Wang, S.-M. Lu, P.-Y. Yang, X.-W. Han, Y.-G. Zhou, J. Am. Chem. Soc. 2003, 125, 10536;
- 3bM. Rueping, A. P. Antonchick, T. Theissmann, Angew. Chem. 2006, 118, 3765;
10.1002/ange.200600191 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3683;
- 3cH.-F. Zhou, Z.-W. Li, Z.-J. Wang, T.-L. Wang, L.-J. Xu, Y.-M. He, Q.-H. Fan, J. Pan, L.-Q. Gu, A. S. C. Chan, Angew. Chem. 2008, 120, 8592; Angew. Chem. Int. Ed. 2008, 47, 8464;
- 3dQ.-S. Guo, D.-M. Du, J. Xu, Angew. Chem. 2008, 120, 771; Angew. Chem. Int. Ed. 2008, 47, 759;
- 3eT.-L. Wang, L.-G. Zhuo, Z.-W. Li, F. Chen, Z.-Y. Ding, Y.-M. He, Q.-H. Fan, J.-F. Xiang, Z.-X. Yu, A. S. C. Chan, J. Am. Chem. Soc. 2011, 133, 9878;
- 3fQ.-A. Chen, K. Gao, Y. Duan, Z.-S. Ye, L. Shi, Y. Yang, Y.-G. Zhou, J. Am. Chem. Soc. 2012, 134, 2442;
- 3gD.-Y. Zhang, C.-B. Yu, M.-C. Wang, K. Gao, Y.-G. Zhou, Tetrahedron Lett. 2012, 53, 2556;
- 3hA. M. Maj, I. Suisse, C. Méliet, C. Hardouin, F. Agbossou-Niedercorn, Tetrahedron Lett. 2012, 53, 4747.
- 4For examples of the asymmetric hydrogenation of isoquinolines, see
- 4aS.-M. Lu, Y.-Q. Wang, X.-W. Han, Y.-G. Zhou, Angew. Chem. 2006, 118, 2318; Angew. Chem. Int. Ed. 2006, 45, 2260;
- 4bL. Shi, Z.-S. Ye, L.-L. Cao, R.-N. Guo, Y. Hu, Y.-G. Zhou, Angew. Chem. 2012, 124, 8411; Angew. Chem. Int. Ed. 2012, 51, 8286.
- 5For selected examples of the asymmetric hydrogenation of quinoxalines, see
- 5aS. Murata, T. Sugimoto, S. Matsuura, Heterocycles 1987, 26, 763;
- 5bC. Bianchini, P. Barbaro, G. Scapacci, E. Farnetti, M. Graziani, Organometallics 1998, 17, 3308;
- 5cC. Bianchini, P. Barbaro, G. Scapacci, J. Organomet. Chem. 2001, 621, 26;
- 5dL. Qiu, F. Y. Kwong, J. Wu, W. H. Lam, S. Chan, W.-Y. Yu, Y.-M. Li, R. Guo, Z. Zhou, A. S. C. Chan, J. Am. Chem. Soc. 2006, 128, 5955;
- 5eW. Tang, L. Xu, Q.-H. Fan, J. Wang, B. Fan, Z. Zhou, K.-H. Lam, A. S. C. Chan, Angew. Chem. 2009, 121, 9299; Angew. Chem. Int. Ed. 2009, 48, 9135;
- 5fN. Mršić, T. Jerphagnon, A. J. Minnaard, B. L. Feringa, J. G. de Vries, Adv. Synth. Catal. 2009, 351, 2549;
- 5gM. Rueping, F. Tato, F. R. Schoepke, Chem. Eur. J. 2010, 16, 2688;
- 5hD. Cartigny, T. Nagano, T. Ayad, J. P. Genêt, T. Ohshima, K. Mashima, V. Ratovelomanana-Vidal, Adv. Synth. Catal. 2010, 352, 1886;
- 5iD.-W. Wang, D.-S. Wang, Q.-A. Chen, Y.-G. Zhou, Chem. Eur. J. 2010, 16, 1133;
- 5jQ.-A. Chen, D.-S. Wang, Y.-G. Zhou, Y. Duan, H.-J. Fan, Y. Yang, Z. Zhang, J. Am. Chem. Soc. 2011, 133, 6126;
- 5kD. Cartigny, F. Berhal, T. Nagano, P. Phanasavath, T. Ayad, P. Genêt, T. Ohshima, K. Mashima, V. Ratovelomanana-Vidal, J. Org. Chem. 2012, 77, 4544.
- 6For selected examples of the hydrogenation of pyridines, see
- 6aF. Glorius, N. Spielkamp, S. Holle, R. Goddard, C. W. Lehmann, Angew. Chem. 2004, 116, 2783; Angew. Chem. Int. Ed. 2004, 43, 2727;
- 6bZ.-S. Ye, M.-W. Chen, Q.-A. Chen, L. Shi, Y. Duan, Y.-G. Zhou, Angew. Chem. 2012, 124, 10328; Angew. Chem. Int. Ed. 2012, 51, 10181.
- 7For recent examples of the asymmetric hydrogenation of indoles/pyrroles, see
- 7aR. Kuwano, K. Sato, T. Kurokawa, D. Karube, Y. J. Ito, J. Am. Chem. Soc. 2000, 122, 7614;
- 7bR. Kuwano, K. Kaneda, T. Ito, K. Sato, T. Kurokawa, Y. Ito, Org. Lett. 2004, 6, 2213;
- 7cR. Kuwano, M. Kashiwabara, Org. Lett. 2006, 8, 2653;
- 7dR. Kuwano, M. Kashiwabara, M. Ohsumi, H. Kusano, J. Am. Chem. Soc. 2008, 130, 808–809;
- 7eN. Mršić, T. Jerphagnon, A. J. Minnaard, B. L. Feringa, J. G. de Vries, Tetrahedron: Asymmetry 2010, 21, 7;
- 7fA. Baeza, A. Pfaltz, Chem. Eur. J. 2010, 16, 2036;
- 7gD.-S. Wang, Q.-A. Chen, W. Li, C.-B. Yu, Y.-G. Zhou, X. J. Zhang, J. Am. Chem. Soc. 2010, 132, 8909;
- 7hD.-S. Wang, J. Tang, Y.-G. Zhou, M.-W. Chen, Y. Duan, G.-F. Jiang, Chem. Sci. 2011, 2, 803;
- 7iY. Duan, M.-W. Chen, Z.-S. Ye, D.-S. Wang, Q.-A. Chen, Y.-G. Zhou, Chem. Eur. J. 2011, 17, 7193;
- 7jY. Duan, M.-W. Chen, Q.-A. Chen, C.-B. Yu, Y.-G. Zhou, Org. Biomol. Chem. 2012, 10, 1235;
- 7kD.-S. Wang, Z.-S. Ye, Q.-A. Chen, Y.-G. Zhou, C.-B. Yu, H.-J. Fan, Y. Duan, J. Am. Chem. Soc. 2011, 133, 8866.
- 8T. Wang, F. Chen, J. Qin, Y.-M. He, Q.-H. Fan, Angew. Chem. 2013, 125, 7313; Angew. Chem. Int. Ed. 2013, 52, 7172.
- 9For selected examples of the hydrogenation of (benzo)thiophenes, see
- 9aC. Bianchini, A. Meli, Acc. Chem. Res. 1998, 31, 109;
- 9bA. F. Borowski, S. Sabo-Etienne, B. Donnadieu, B. Chaudret, Organometallics 2003, 22, 4803;
- 9cS. Urban, B. Beiring, N. Ortega, D. Paul, F. Glorius, J. Am. Chem. Soc. 2012, 134, 15241.
- 10For selected examples of the hydrogenation of benzofurans, see
- 10aM. Maris, W.-R. Huck, T. Mallat, A. Baiker, J. Catal. 2003, 219, 52;
- 10bS. Kaiser, S. P. Smidt, A. Pfaltz, Angew. Chem. 2006, 118, 5318;
10.1002/ange.200601529 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5194;
- 10cN. Ortega, S. Urban, B. Beiring, F. Glorius, Angew. Chem. 2012, 124, 1742;
10.1002/ange.201107811 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1710;
- 10dN. Ortega, B. Beiring, S. Urban, F. Glorius, Tetrahedron 2012, 68, 5185.
- 11For the asymmetric hydrogenation of arenes, see
- 11aR. Kuwano, R. Morioka, M. Kashiwabara, N. Kameyama, Angew. Chem. 2012, 124, 4212;
10.1002/ange.201201153 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 4136;
- 11bS. Urban, N. Ortega, F. Glorius, Angew. Chem. 2011, 123, 3887; Angew. Chem. Int. Ed. 2011, 50, 3803.
- 12For selected examples of the hydrogenation of furans, see
- 12aT. Ohta, T. Miyake, N. Seido, H. Kumobayashi, H. Takaya, J. Org. Chem. 1995, 60, 357;
- 12bM. Studer, C. Wedemeyer-Exl, F. Spindler, H.-U. Blaser, Monatsh. Chem. 2000, 131, 1335;
- 12cP. Feiertag, M. Albert, U. Nettekoven, F. Spindler, Org. Lett. 2006, 8, 4133.
- 13D. Zhao, B. Beiring, F. Glorius, Angew. Chem. 2013, 125, 8612; Angew. Chem. Int. Ed. 2013, 52, 8454.
- 14N. Ortega, D.-T. D. Tang, S. Urban, D. Zhao, F. Glorius, Angew. Chem. 2013, 125, 9678;
10.1002/ange.201302218 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 9500.
- 15Throughout this study, the minor trans isomers of 2,5-disubstituted tetrahydrofurans were observed as single enantiomers.
- 16C. Hansch, A. Leo, R. W. Taft, Chem. Rev. 1991, 91, 165.
- 17M. Tiecco, L. Testaferri, L. Bagnoli, V. Purgatorio, A. Temperini, F. Marini, C. Santi, Tetrahedron: Asymmetry 2004, 15, 405.
- 18Our attempt to hydrogenate 2-phenylfuran as a representative monosubstituted furan afforded the corresponding product with full conversion, although with a low ee value (20 %).