Decay of Iron(V) Nitride Complexes By a NN Bond-Coupling Reaction in Solution: A Combined Spectroscopic and Theoretical Analysis†
We thank Petra Höfer for her assistance with the electrochemical measurements, Dr. Wolfgang Schrader for his support in the mass spectrometry study and the Max-Planck society for financial support. This work was initiated in the framework of the SFB 813 (University of Bonn).
Graphical Abstract
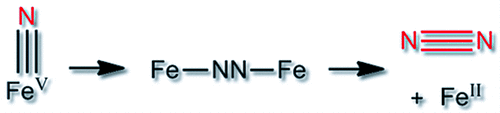
Always look on the bright azide of life: the qualitative formation of dinitrogen results from the decay of FeV nitride complexes and is investigated by a combination of spectroscopy, spectrometry, and theory. New insight is obtained in the photolysis of iron azide complexes, which is crucial for the formation of iron nitrides.
Abstract
Cryogenically trapped FeV nitride complexes with cyclam-based ligands were found to decay by bimolecular reactions, forming exclusively FeII compounds. Characterization of educts and products by Mössbauer spectroscopy, mass spectrometry, and spectroscopy-oriented DFT calculations showed that the reaction mechanism is reductive nitride coupling and release of dinitrogen (2 FeVN→FeII-NN-FeII→2 FeII+N2). The reaction pathways, representing an “inverse” of the Haber–Bosch reaction, were computationally explored in detail, also to judge the feasibility of yielding catalytically competent FeV(N). Implications for the photolytic cleavage of FeIII azides used to generate high-valent Fe nitrides are discussed.