Copper-Catalyzed Regioselective ortho CH Cyanation of Vinylarenes†
Yang Yang
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen L. Buchwald
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)Search for more papers by this authorYang Yang
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stephen L. Buchwald
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)
Department of Chemistry, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139 (USA)Search for more papers by this authorWe thank the National Institutes of Health (GM46059) for financial support. We are grateful to Dr. Aaron C. Sather (MIT), Dr. Yi-Ming Wang (MIT), and Dr. Daniel T. Cohen (MIT) for insightful discussions and help with the preparation of this manuscript. MIT has patents on some of the ligands used in this study from which S.L.B. as well as current or former co-workers receive royalty payments. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Graphical Abstract
Reaction combo: A combined copper-catalyzed hydroborylation/ortho CH cyanation of vinylarenes is described, thus allowing the selective functionalization of vinylarenes and featuring unique site selectivity. The reaction leads to ortho-selective CH functionalization of arenes and anti-Markovnikov hydrofunctionalization of the pendant olefin (see scheme; Pin=pinacolato, Ts=4-toluenesulfonyl).
Abstract
A copper-based catalytic technique for the regioselective ortho CH cyanation of vinylarenes has been developed. This method provides an effective means for the selective functionalization of vinylarene derivatives. A copper-catalyzed cyanative dearomatization mechanism is proposed to account for the regiochemical course of this reaction.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201402449_sm_miscellaneous_information.pdf31.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected reviews on directed CH activation:
- 1aF. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda, N. Chatani, S. Murai, Bull. Chem. Soc. Jpn. 1995, 68, 62;
- 1bC.-H. Jun, J.-B. Hong, D.-Y. Lee, Synlett 1999, 1;
- 1cO. Daugulis, H.-Q. Do, D. Shabashov, Acc. Chem. Res. 2009, 42, 1074;
- 1dM. Albrecht, Chem. Rev. 2010, 110, 576;
- 1eD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 1fC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215;
- 1gK. M. Engle, T.-S. Mei, M. Wasa, J.-Q. Yu, Acc. Chem. Res. 2012, 45, 788;
- 1hS. R. Neufeldt, M. S. Sanford, Acc. Chem. Res. 2012, 45, 936.
- 2Selected reviews on other CH functionalizations:
- 2aI. A. Mkhalid, J. H. Barnard, T. B. Marder, J. M. Murphy, J. F. Hartwig, Chem. Rev. 2010, 110, 890;
- 2bA. E. Wendlandt, A. M. Suess, S. S. Stahl, Angew. Chem. 2011, 123, 11256;
10.1002/ange.201103945 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11062;
- 2cH. M. L. Davies, D. Morton, Chem. Soc. Rev. 2011, 40, 1857.
- 3For a palladium-catalyzed CH activation directed by an ortho allylic CC bond, see: P. Gandeepan, C.-H. Cheng, J. Am. Chem. Soc. 2012, 134, 5738. Styrenes were unreactive under these reaction conditions.
- 4M. Tobisu, I. Hyodo, M. Onoe, N. Chatani, Chem. Commun. 2008, 6013.
- 5R. C. Larock, Comprehensive Organic Transformations, Wiley-VCH, New York, 1988.
- 6For a review, see: M. Beller, J. Seavad, A. Tillack, H. Jiao, Angew. Chem. 2004, 116, 3448;
10.1002/ange.200300616 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3368; For a recent example: G. Dong, P. Teo, Z. K. Wickens, R. H. Grubbs, Science 2011, 333, 1609.
- 7For example, we have recently demonstrated the utility of benzylcopper intermediates generated from hydrocupration of styrenes in an enantioselective amination process: S. Zhu, N. Niljianskul, S. L. Buchwald, J. Am. Chem. Soc. 2013, 135, 15746.
- 8R. Zhu, S. L. Buchwald, Angew. Chem. 2013, 125, 12887; Angew. Chem. Int. Ed. 2013, 52, 12655.
- 9Examples of borocupration of styrenes:
- 9aD. S. Laitar, E. Y. Tsui, J. P. Sadighi, Organometallics 2006, 25, 2405;
- 9bV. Lillo, M. R. Fructos, J. Ramírez, A. A. C. Braga, F. Maseras, M. M. Díaz-Requejo, P. J. Pérez, E. Fernández, Chem. Eur. J. 2007, 13, 2614;
- 9cY. Lee, A. H. Hoveyda, J. Am. Chem. Soc. 2009, 131, 3160;
- 9dC. Zhong, S. Kunii, Y. Kosaka, M. Sawamura, H. Ito, J. Am. Chem. Soc. 2010, 132, 11440;
- 9eH. Ito, T. Toyoda, M. Sawamura, J. Am. Chem. Soc. 2010, 132, 5990;
- 9fR. Corberán, N. W. Mszar, A. H. Hoveyda, Angew. Chem. 2011, 123, 7217;
10.1002/ange.201102398 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7079;
- 9gN. Matsuda, K. Hirano, T. Satoh, M. Miura, J. Am. Chem. Soc. 2013, 135, 11440; Computational studies:
- 9hL. Dang, H. Zhao, Z. Lin, T. B. Marder, Organometallics 2007, 26, 2824.
- 10NCTS (2) is a safe and bench-stable crystalline compound which can be easily prepared from tosyl chloride and phenylurea. For the synthesis of NCTS:
- 10aF. Kurzer, J. Chem. Soc. 1949, 1034;
- 10bF. Kurzer, J. Chem. Soc. 1949, 3029; For the application of NCTS in electrophilic cyanation:
- 10cP. Anbarasan, H. Neumann, M. Beller, Angew. Chem. 2011, 123, 539;
10.1002/ange.201006044 Google Scholar
- 10dAngew. Chem. Int. Ed. 2011, 50, 519;
- 10eP. Anbarasan, H. Neumann, M. Beller, Chem. Eur. J. 2011, 17, 4271;
- 10fY. Yang, Y. Zhang, J. Wang, Org. Lett. 2011, 13, 5068;
- 10gT.-J. Gong, B. Xiao, W.-M. Cheng, W. Su, J. Xu, Z.-J. Liu, L. Liu, Y. Fu, J. Am. Chem. Soc. 2013, 135, 10630;
- 10hM. Chaitanya, D. Yadagiri, P. Anbarasan, Org. Lett. 2013, 15, 4960;
- 10iW. Liu, L. Ackermann, Chem. Commun. 2014, 50, 1878.
- 11J. P. Wolfe, S. L. Buchwald, Angew. Chem. 1999, 111, 2570;
10.1002/(SICI)1521-3757(19990816)111:16<2570::AID-ANGE2570>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2413.10.1002/(SICI)1521-3773(19990816)38:16<2413::AID-ANIE2413>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 12For a detailed ligand evaluation, see the Supporting Information.
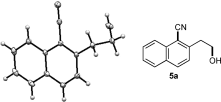
- 13The structure of 5 a was confirmed by X-ray diffraction analysis. CCDC 986749 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 14K. Kubota, E. Yamamoto, H. Ito, J. Am. Chem. Soc. 2013, 135, 2635.
- 15N. C. Bruno, M. T. Tudge, S. L. Buchwald, Chem. Sci. 2013, 4, 916.
- 16S. Caddick, A. K. K. Haynes, D. B. Judd, M. R. V. Williams, Tetrahedron Lett. 2000, 41, 3513.
- 17Z. P. Demko, K. B. Sharpless, J. Org. Chem. 2001, 66, 7945.
- 18
- 18aJ. M. Brunel, Chem. Rev. 2005, 105, 857;
- 18bY. Chen, S. Yekata, A. K. Yudin, Chem. Rev. 2003, 103, 3155;
- 18cM. Berthod, G. Mignani, G. Woodward, M. Lemaire, Chem. Rev. 2005, 105, 1801.
- 19For reviews on alternative methods to prepare fully substituted arenes, see:
- 19aP. R. Chopade, J. Louie, Adv. Synth. Catal. 2006, 348, 2307;
- 19bW. A. L. van Otterlo, C. B. de Koning, Chem. Rev. 2009, 109, 3743.
- 20For an example, see: D. Kalyani, A. R. Dick, W. Q. Anani, M. S. Sanford, Tetrahedron 2006, 62, 11483.
- 21For a recent review on the chemistry of benzylmetal species, see: B. M. Trost, L. C. Czabaniuk, Angew. Chem. 2014, 126, 2868;
10.1002/ange.201305972 Google ScholarAngew. Chem. Int. Ed. 2014, 53, 2826.
- 22For palladium-catalyzed nucleophilic aromatic CH functionalization processes involving dearomatization mechanism, see:
- 22aallylation: M. Bao, H. Nakamura, Y. Yamamoto, J. Am. Chem. Soc. 2001, 123, 759. Related computational studies: A. Ariafard, A. Lin, J. Am. Chem. Soc. 2006, 128, 13010;
- 22bamination: S. Zhang, Y. Wang, X. Feng, M. Bao, J. Am. Chem. Soc. 2012, 134, 5492; Related computational studies: H. Xie, H. Zhang, Z. Lin, Organometallics 2013, 32, 2336.
- 23For a copper-catalyzed 1,3-hologen migration by an oxidative addition mechanism, see: R. D. Grigg, R. Van Hoveln, J. M. Schomaker, J. Am. Chem. Soc. 2012, 134, 16131.
- 24An alternative mechanism involving oxidative addition to a copper(III) intermediate is also possible, see Ref. [7].





