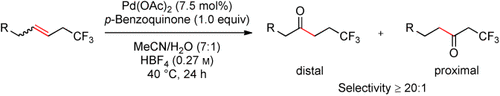Rapid Access to β-Trifluoromethyl-Substituted Ketones: Harnessing Inductive Effects in Wacker-Type Oxidations of Internal Alkenes†
Michael M. Lerch
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125 (USA)
Search for more papers by this authorDr. Bill Morandi
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125 (USA)
Search for more papers by this authorZachary K. Wickens
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Robert H. Grubbs
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125 (USA)
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125 (USA)Search for more papers by this authorMichael M. Lerch
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125 (USA)
Search for more papers by this authorDr. Bill Morandi
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125 (USA)
Search for more papers by this authorZachary K. Wickens
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Robert H. Grubbs
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125 (USA)
Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125 (USA)Search for more papers by this authorWe gratefully acknowledge financial support from the King Abdullah University of Science and Technology Centre in Development, King Fahd University of Petroleum and Minerals, and the NSF. Furthermore, we thank the Gordon and Betty Moore Foundation, the SNSF for a fellowship to B.M., and the Swiss Study Foundation for a fellowship to M.M.L.
Graphical Abstract
Synthetically highly desirable β-trifluoromethyl-substituted ketones can be rapidly accessed by a trifluoromethyl-directed Wacker oxidation starting from alkenes bearing an allylic trifluoromethyl group (see scheme). This effect seems to be dominantly inductive and can override coordinative effects. The reaction has a broad substrate scope and affords products in high yields and very high regioselectivity.
Abstract
We present a practical trifluoromethyl-directed Wacker-type oxidation of internal alkenes that enables rapid access to β-trifluoromethyl-substituted ketones. Allylic trifluoromethyl-substituted alkenes bearing a wide range of functional groups can be oxidized in high yield and regioselectivity. The distance dependence of the regioselectivity was established by systematic variation of the number of methylene units between the double bond and the trifluoromethyl group. The regioselectivity enforced by traditional directing groups could even be reversed by introduction of a competing trifluoromethyl group. Besides being a new powerful synthetic method to prepare fluorinated molecules, this work directly probes the role of inductive effects on nucleopalladation events.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201404712_sm_miscellaneous_information.pdf4.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected books on fluorine-containing molecules and their chemistry, see
- 1aP. Kirsch in Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications, Wiley-VCH, Weinheim, 2013;
10.1002/9783527651351 Google Scholar
- 1bT. Hiyama in Organofluorine Compounds: Chemistry and Applications, Springer, Berlin, 2000;
10.1007/978-3-662-04164-2 Google Scholar
- 1cK. Uneyama in Organofluorine Chemistry, Blackwell, Oxford, 2006.
10.1002/9780470988589 Google Scholar
- 2For a selection of books and reviews of fluorine in medicinal chemistry and pharmacology, see
- 2aI. Ojima in Fluorine in Medicinal Chemistry and Chemical Biology (Ed.: ), Wiley-Blackwell, Chichester, 2009;
10.1002/9781444312096 Google Scholar
- 2bJ. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok, H. Liu, Chem. Rev. 2013, 113, 2432–2506;
- 2cK. Müller, C. Faeh, F. Diederich, Science 2007, 317, 1881–1886;
- 2dS. Purser, P. R. Moore, S. Swallow, V. Gouverneur, Chem. Soc. Rev. 2008, 37, 320–330;
- 2eD. O’Hagan, Chem. Soc. Rev. 2008, 37, 308–319.
- 3For an introduction to fluorine in agrochemistry, see P. Jeschke in Modern Methods in Crop Protection Research, Wiley-VCH, Weinheim, 2012, pp. 73–128.
10.1002/9783527655908 Google Scholar
- 4For a selective introduction to fluorine’s role in materials chemistry, see
- 4aR. Berger, G. Resnati, P. Metrangolo, E. Weber, J. Hulliger, Chem. Soc. Rev. 2011, 40, 3496–3508;
- 4bF. Babudri, G. M. Farinola, F. Naso, R. Ragni, Chem. Commun. 2007, 1003–1022.
- 5For recent reviews on methods to access trifluoromethylated compounds in general, see
- 5aM. Shimizu, T. Hiyama, Angew. Chem. 2005, 117, 218–234; Angew. Chem. Int. Ed. 2005, 44, 214–231;
- 5bM. Schlosser, Angew. Chem. 2006, 118, 5558–5572;
10.1002/ange.200600449 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5432–5446;
- 5cT. Furuya, A. S. Kamlet, T. Ritter, Nature 2011, 473, 470–477;
- 5dT. Liang, C. N. Neumann, T. Ritter, Angew. Chem. 2013, 125, 8372–8423; Angew. Chem. Int. Ed. 2013, 52, 8214–8264;
- 5eO. A. Tomashenko, V. V. Grushin, Chem. Rev. 2011, 111, 4475–4521;
- 5fM. C. Pacheco, S. Purser, V. Gouverneur, Chem. Rev. 2008, 108, 1943–1981;
- 5gN. Shibata, S. Mizuta, H. Kawai, Tetrahedron: Asymmetry 2008, 19, 2633–2644;
- 5hA. Studer, Angew. Chem. 2012, 124, 9082–9090; Angew. Chem. Int. Ed. 2012, 51, 8950–8958;
- 5iJ. Nie, H.-C. Guo, D. Cahard, J.-A. Ma, Chem. Rev. 2010, 110, 455–529.
- 6For copper-catalyzed trifluoromethylation reactions of terminal alkenes, see
- 6aA. T. Parsons, S. L. Buchwald, Angew. Chem. 2011, 123, 9286–9289; Angew. Chem. Int. Ed. 2011, 50, 9120–9123;
- 6bX. Wang, Y. Ye, S. Zhang, J. Feng, Y. Xu, Y. Zhang, J. Wang, J. Am. Chem. Soc. 2011, 133, 16410–16413;
- 6cJ. Xu, Y. Fu, D.-F. Luo, Y.-Y. Jiang, B. Xiao, Z.-J. Liu, T.-J. Gong, L. Liu, J. Am. Chem. Soc. 2011, 133, 15300–15303;
- 6dL. Chu, F.-L. Qing, Org. Lett. 2012, 14, 2106–2109; for a selection of further recent reports, see
- 6eA. E. Allen, D. W. C. MacMillan, J. Am. Chem. Soc. 2010, 132, 4986–4987;
- 6fP. Novák, A. Lishchynskyi, V. V. Grushin, J. Am. Chem. Soc. 2012, 134, 16167–16170;
- 6gS. Mizuta, S. Verhoog, K. M. Engle, T. Khotavivattana, M. O’Duill, K. Wheelhouse, G. Rassias, M. Médebielle, V. Gouverneur, J. Am. Chem. Soc. 2013, 135, 2505–2508;
- 6hH. Morimoto, T. Tsubogo, N. D. Litvinas, J. F. Hartwig, Angew. Chem. 2011, 123, 3877–3882;
10.1002/ange.201100633 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 3793–3798;
- 6iL. Chu, F.-L. Qing, Org. Lett. 2010, 12, 5060–5063;
- 6jX. Wang, L. Truesdale, J.-Q. Yu, J. Am. Chem. Soc. 2010, 132, 3648–3649.
- 7For alternative methods to access β-trifluoromethylated carbonyl compounds, see (by Friedel—Crafts alkylations)
- 7aG. Blay, I. Fernández, M. C. Muñoz, J. R. Pedro, C. Vila, Chem. Eur. J. 2010, 16, 9117–9122;
- 7bY. Huang, E. Tokunaga, S. Suzuki, M. Shiro, N. Shibata, Org. Lett. 2010, 12, 1136–1138;
- 7cL. Wen, Q. Shen, X. Wan, L. Lu, J. Org. Chem. 2011, 76, 2282–2285; by Michael additions to β-trifluoromethyl enones, see
- 7dM. Yasuda, K. Chiba, N. Ohigashi, Y. Katoh, A. Baba, J. Am. Chem. Soc. 2003, 125, 7291–7300;
- 7eT. Konno, T. Tanaka, T. Miyabe, A. Morigaki, T. Ishihara, Tetrahedron Lett. 2008, 49, 2106–2110;
- 7fO. Marrec, J. Borrini, T. Billard, B. R. Langlois, Synlett 2009, 1241–1244;
- 7gO. Marrec, C. Christophe, T. Billard, B. Langlois, J.-P. Vors, S. Pazenok, Adv. Synth. Catal. 2010, 352, 2825–2830;
- 7hH. Kawai, S. Okusu, E. Tokunaga, H. Sato, M. Shiro, N. Shibata, Angew. Chem. 2012, 124, 5043–5046; Angew. Chem. Int. Ed. 2012, 51, 4959–4962; by trifluoromethylation of enones, see
- 7iA. A. Zemtsov, V. V. Levin, A. D. Dilman, M. I. Struchkova, P. A. Belyakov, V. A. Tartakovsky, Tetrahedron Lett. 2009, 50, 2998–3000.
- 8For the initial development of the Wacker process, see
- 8aJ. Smidt, W. Hafner, R. Jira, J. Sedlmeier, R. Sieber, R. Rüttinger, H. Kojer, Angew. Chem. 1959, 71, 176–182;
- 8bJ. Smidt, W. Hafner, R. Jira, R. Sieber, J. Sedlmeier, A. Sabel, Angew. Chem. 1962, 74, 93–102; Angew. Chem. Int. Ed. 1962, 1, 80–88; for the development of the Tsuji–Wacker procedure, see
- 8cJ. Tsuji in Palladium Reagents and Catalysts: New Perspectives for the 21st Century, 2nd ed., Wiley, 2004;
10.1002/0470021209 Google Scholar
- 8dJ. Tsuji, Synthesis 1984, 369–384, and references therein; for a mechanistic discussion, see
- 8eJ. A. Keith, P. M. Henry, Angew. Chem. 2009, 121, 9200–9212;
10.1002/ange.200902194 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9038–9049;
- 8fJ.-E. Bäckvall, B. Akermark, S. O. Ljunggren, J. Am. Chem. Soc. 1979, 101, 2411–2416;
- 8gR. I. McDonald, G. Liu, S. S. Stahl, Chem. Rev. 2011, 111, 2981–3019;
- 8hM. J. Gaunt, J. Yu, J. B. Spencer, Chem. Commun. 2001, 1844–1845; for the usage of O2 as terminal oxidant, see
- 8iA. N. Campbell, S. S. Stahl, Acc. Chem. Res. 2012, 45, 851–863;
- 8jC. N. Cornell, M. S. Sigman, Inorg. Chem. 2007, 46, 1903–1909;
- 8kK. M. Gligorich, M. S. Sigman, Chem. Commun. 2009, 3854–3867.
- 9For the oxidation of internal alkenes under Wacker-type conditions, see
- 9aD. G. Miller, D. D. M. Wayner, J. Org. Chem. 1990, 55, 2924–2927;
- 9bT. Mitsudome, K. Mizumoto, T. Mizugaki, K. Jitsukawa, K. Kaneda, Angew. Chem. 2010, 122, 1260–1262;
10.1002/ange.200905184 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 1238–1240;
- 9cB. Morandi, Z. K. Wickens, R. H. Grubbs, Angew. Chem. 2013, 125, 3016–3020;
10.1002/ange.201209541 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 2944–2948;
- 9dR. J. DeLuca, J. L. Edwards, L. D. Steffens, B. W. Michel, X. Qiao, C. Zhu, S. P. Cook, M. S. Sigman, J. Org. Chem. 2013, 78, 1682–1686;
- 9eT. Mitsudome, S. Yoshida, T. Mizugaki, K. Jitsukawa, K. Kaneda, Angew. Chem. 2013, 125, 6077–6080;
10.1002/ange.201301611 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 5961–5964;
- 9fT. Mitsudome, S. Yoshida, Y. Tsubomoto, T. Mizugaki, K. Jitsukawa, K. Kaneda, Tetrahedron Lett. 2013, 54, 1596–1598;
- 9gB. Morandi, Z. K. Wickens, R. H. Grubbs, Angew. Chem. 2013, 125, 9933–9936;
10.1002/ange.201303587 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 9751–9754.
- 10In some cases, the introduction of suitable coordinating groups proximal to internal alkenes promoted Wacker oxidation (very substrate dependent), see
- 10aJ. Tsuji, H. Nagashima, K. Hori, Tetrahedron Lett. 1982, 23, 2679–2682;
- 10bE. Keinan, K. K. Seth, R. Lamed, J. Am. Chem. Soc. 1986, 108, 3474–3480;
- 10cS.-K. Kang, K.-Y. Jung, J.-U. Chung, E.-Y. Namkoong, T.-H. Kim, J. Org. Chem. 1995, 60, 4678–4679;
- 10dB. M. Trost, T. L. Calkins, Tetrahedron Lett. 1995, 36, 6021–6024;
- 10eY. Sato, N. Saito, M. Mori, J. Org. Chem. 2002, 67, 9310–9317;
- 10fP. R. Skaanderup, R. Madsen, J. Org. Chem. 2003, 68, 2115–2122;
- 10gS. B. Narute, N. C. Kiran, C. V. Ramana, Org. Biomol. Chem. 2011, 9, 5469–5475.
- 11Early reports indicated that the use of Pd(OAc)2 in chloride-free conditions resulted in precipitation of Pd0. This limitation, however, could be overcome by the addition of strong acid (e.g. HClO4 or HBF4), see
- 11aJ. E. Bäckvall, R. B. Hopkins, Tetrahedron Lett. 1988, 29, 2885–2888;
- 11bJ.-E. Bäckvall, R. B. Hopkins, H. Grennberg, M. Mader, A. K. Awasthi, J. Am. Chem. Soc. 1990, 112, 5160–5166.
- 12The lower reactivity of these substrates compared to unfunctionalized internal alkenes is likely due to the decreased electron density of the double bond bearing a neighboring, electron-withdrawing trifluoromethyl group, which slows down the rate of oxidation.
- 13For a selection of references about using the trifluoromethyl group to control reactions and selectivity, see
- 13aS. Fioravanti, D. Colantoni, L. Pellacani, P. A. Tardella, J. Org. Chem. 2005, 70, 3296–3298;
- 13bA. Fu, W. Meng, H. Li, J. Nie, J.-A. Ma, Org. Biomol. Chem. 2014, 12, 1908–1918;
- 13cG. Hornyák, J. Fetter, G. Németh, L. Poszávácz, G. Simig, J. Fluorine Chem. 1997, 84, 49–51;
- 13dJ. Liu, K. J. Boarman, Chem. Commun. 2005, 340–341;
- 13eJ. Liu, N. L. Wendt, K. J. Boarman, Org. Lett. 2005, 7, 1007–1010;
- 13fJ. Nie, G.-W. Zhang, L. Wang, A. Fu, Y. Zheng, J.-A. Ma, Chem. Commun. 2009, 2356–2358;
- 13gA. Vargas, F. Hoxha, N. Bonalumi, T. Mallat, A. Baiker, J. Catal. 2006, 240, 203–212;
- 13hI. Ojima, K. Kato, M. Okabe, T. Fuchikami, J. Am. Chem. Soc. 1987, 109, 7714–7720;
- 13iI. Ojima, M. Okabe, K. Kato, H. B. Kwon, I. T. Horvath, J. Am. Chem. Soc. 1988, 110, 150–157;
- 13jT. Katagiri, S. Yamaji, M. Handa, M. Irie, K. Uneyama, Chem. Commun. 2001, 2054–2055;
- 13kV. A. Soloshonok, T. Hayashi, K. Ishikawa, N. Nagashima, Tetrahedron Lett. 1994, 35, 1055–1058;
- 13lV. A. Soloshonok, D. V. Avilov, V. P. Kukhar, Tetrahedron 1996, 52, 12433–12442;
- 13mX. Lin, F.-L. Qing, Org. Lett. 2013, 15, 4478–4481.
- 14For direct comparison, substrates with an allylic trifluoromethyl group and an allylic directing group (-OBn, -OBz, -OFur) were designed and tested. However, these substrates were oxidized in very low conversion.
- 15Compounds containing a vinylic trifluoromethyl group were found to not react under the optimized conditions.
- 16We hypothesize that this selectivity is derived from a combination of the higher stability of the positive charge build up in the transition state at the distal position and the stabilization of negative charge build up at the proximal position effected by the trifluoromethyl group.
- 17For reports that achieved anti-Markovnikov selectivity in Wacker oxidation, see
- 17aJ. Muzart, Tetrahedron 2007, 63, 7505;
- 17bB. W. Michel, A. M. Camelio, C. N. Cornell, M. S. Sigman, J. Am. Chem. Soc. 2009, 131, 6076–6077;
- 17cB. Weiner, A. Baeza, T. Jerphagnon, B. L. Feringa, J. Am. Chem. Soc. 2009, 131, 9473–9474;
- 17dB. W. Michel, J. R. McCombs, A. Winkler, M. S. Sigman, Angew. Chem. 2010, 122, 7470–7473;
10.1002/ange.201004156 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7312–7315;
- 17eM. S. Sigman, E. W. Werner, Acc. Chem. Res. 2011, 44, 874–884;
- 17fG. Dong, P. Teo, Z. K. Wickens, R. H. Grubbs, Science 2011, 333, 1609–1612;
- 17gP. Teo, Z. K. Wickens, G. Dong, R. H. Grubbs, Org. Lett. 2012, 14, 3237–3239;
- 17hJ. J. Dong, M. Fañanás-Mastral, P. L. Alsters, W. R. Browne, B. L. Feringa, Angew. Chem. 2013, 125, 5671–5675; Angew. Chem. Int. Ed. 2013, 52, 5561–5565;
- 17iZ. K. Wickens, B. Morandi, R. H. Grubbs, Angew. Chem. 2013, 125, 11467–11470;
10.1002/ange.201306756 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 11257–11260;
- 17jZ. K. Wickens, K. Skakuj, B. Morandi, R. H. Grubbs, J. Am. Chem. Soc. 2014, 136, 890–893.