Catalytically Active and Spectator Ce3+ in Ceria-Supported Metal Catalysts†
We thank V. Scagnoli (Paul Scherrer Institute), A. Guda and A. Bugaev (Rostov-on-Don Federal University) for their help with the data analysis. We thank D. Ferri and V. Marchionni for help with in situ setup. We thank Dr. F. Krumeich for the STEM measurements. We thank P. Ascher, E. de Boni, L. Baeni, and M. Birri (Paul Scherrer Institute) for technical help. We thank M. Rothensteiner (Paul Scherrer Institute), U. Hartfelder, and R. Bessa Duarte (ETH Zurich) for help with data collection during beamtime. We thank the Swiss Light Source for providing beamtime at the SuperXAS beamline. Financial support of the Swiss National Science Foundation (project number 200021_140750) for R.K. is gratefully acknowledged.
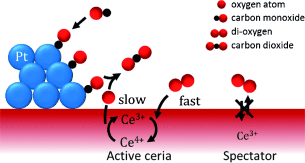
Graphical Abstract
Short-lived Ce3+ is the active and kinetically relevant intermediate during catalytic CO oxidation on a ceria-supported metal catalyst, whereas long-lived Ce3+ species are inactive spectators. This was shown by in situ resonant X-ray emission spectroscopy and quantitative correlation between the initial rate of Ce3+ formation under transient conditions and the overall CO oxidation rate.
Abstract
Identification of active species and the rate-determining reaction steps are crucial for optimizing the performance of oxygen-storage materials, which play an important role in catalysts lowering automotive emissions, as electrode materials for fuel cells, and as antioxidants in biomedicine. We demonstrated that active Ce3+ species in a ceria-supported platinum catalyst during CO oxidation are short-lived and therefore cannot be observed under steady-state conditions. Using time-resolved resonant X-ray emission spectroscopy, we quantitatively correlated the initial rate of Ce3+ formation under transient conditions to the overall rate of CO oxidation under steady-state conditions and showed that ceria reduction is a kinetically relevant step in CO oxidation, whereas a fraction of Ce3+ was present as spectators. This approach can be applied to various catalytic processes involving oxygen-storage materials and reducible oxides to distinguish between redox and nonredox catalytic mechanisms.