Rapid Autoxidation Forms Highly Oxidized RO2 Radicals in the Atmosphere†
We thank K. Pielok, R. Gräfe, and A. Rohmer for technical assistance and the tofTools team for providing data analysis software. This work was funded in part by the European Commission (FP7-ENV-2010-265148), Academy of Finland (CoE project 1118615 and project 251427), and European research council (ATMNUCLE, grant 227463).
Graphical Abstract
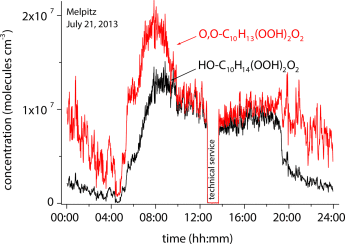
Not only in the solution phase: Highly oxidized RO2 radicals in the atmosphere are rapidly formed by autoxidation initiated by the reaction of O3 and OH radicals with biogenic emissions such as limonene and α-pinene. Field measurements (see picture) confirm experimental findings from a flow-tube study. The closed-shell products from this process represent important aerosol constituents influencing aerosol–cloud–climate interactions.
Abstract
Gas-phase oxidation routes of biogenic emissions, mainly isoprene and monoterpenes, in the atmosphere are still the subject of intensive research with special attention being paid to the formation of aerosol constituents. This laboratory study shows that the most abundant monoterpenes (limonene and α-pinene) form highly oxidized RO2 radicals with up to 12 O atoms, along with related closed-shell products, within a few seconds after the initial attack of ozone or OH radicals. The overall process, an intramolecular ROO→QOOH reaction and subsequent O2 addition generating a next R′OO radical, is similar to the well-known autoxidation processes in the liquid phase (QOOH stands for a hydroperoxyalkyl radical). Field measurements show the relevance of this process to atmospheric chemistry. Thus, the well-known reaction principle of autoxidation is also applicable to the atmospheric gas-phase oxidation of hydrocarbons leading to extremely low-volatility products which contribute to organic aerosol mass and hence influence the aerosol–cloud–climate system.