Facile Synthesis of Polycyclic Pentalenes with Enhanced Hückel Antiaromaticity
Hiroya Oshima
Department of Chemistry, Graduate School of Science and Integrated Research Consortium on Chemical Sciences (IRCCS), Nagoya University, Furo, Chikusa, Nagoya, 464-8602 Japan
Search for more papers by this authorProf. Dr. Aiko Fukazawa
Department of Chemistry, Graduate School of Science and Integrated Research Consortium on Chemical Sciences (IRCCS), Nagoya University, Furo, Chikusa, Nagoya, 464-8602 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Shigehiro Yamaguchi
Department of Chemistry, Graduate School of Science and Integrated Research Consortium on Chemical Sciences (IRCCS), Nagoya University, Furo, Chikusa, Nagoya, 464-8602 Japan
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya, 464-8602 Japan
Search for more papers by this authorHiroya Oshima
Department of Chemistry, Graduate School of Science and Integrated Research Consortium on Chemical Sciences (IRCCS), Nagoya University, Furo, Chikusa, Nagoya, 464-8602 Japan
Search for more papers by this authorProf. Dr. Aiko Fukazawa
Department of Chemistry, Graduate School of Science and Integrated Research Consortium on Chemical Sciences (IRCCS), Nagoya University, Furo, Chikusa, Nagoya, 464-8602 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Shigehiro Yamaguchi
Department of Chemistry, Graduate School of Science and Integrated Research Consortium on Chemical Sciences (IRCCS), Nagoya University, Furo, Chikusa, Nagoya, 464-8602 Japan
Institute of Transformative Bio-Molecules (WPI-ITbM), Nagoya University, Furo, Chikusa, Nagoya, 464-8602 Japan
Search for more papers by this authorGraphical Abstract
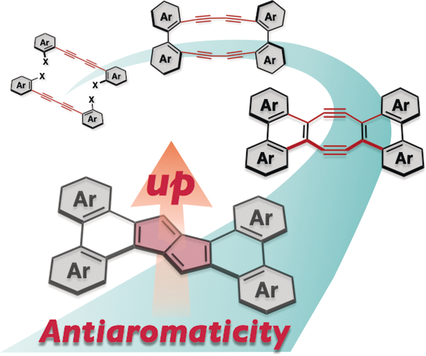
Polycyclic pentalenes with phenanthrene-type π extensions were prepared from 1,4-bis(bromoaryl)-1,3-butadiynes by successive transannular cyclizations of the in situ generated tetrakisdehydro[16]annulenes. These polycyclic pentalenes show not only high thermal stability, but also intriguing absorption and redox properties, due to the pronounced Hückel antiaromaticity.
Abstract
Pentalenes represent highly reactive Hückel antiaromatics with 8π electrons. Usually, pentalenes are stabilized by incorporation of two benzene rings in a fused fashion. In dibenzo[a,e]pentalenes, however, the high aromaticity of the fused benzene rings compromises the inherent antiaromaticity of the pentalene core. Herein, we disclose that this forfeited antiaromaticity can be restored by fusing four additional aromatic rings onto the peripheral positions of dibenzo[a,e]pentalenes. Such polycyclic pentalenes were prepared by successive transannular cyclizations via in situ-generated tetrakisdehydro[16]annulenes. The thus obtained compounds showed intriguing properties, for example, characteristic absorptions in the visible-to-near-infrared (NIR) region and low reduction potentials. These results hence afford a design principle to produce highly antiaromatic yet stable pentalenes. The antiaromaticity of the pentalene core can be widely tuned via the degree of aromaticity of the peripherally fused rings.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201611344-sup-0001-misc_information.pdf10.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1 Aromaticity and Antiaromaticity: Electronic and Structural Aspects (Eds.: ), Wiley-VCH, Weinheim, 1994.
- 2
- 2aD. T. Chase, A. G. Fix, S. J. Kang, B. D. Rose, C. D. Weber, Y. Zhong, L. N. Zakharov, M. C. Lonergan, C. Nuckolls, M. M. Haley, J. Am. Chem. Soc. 2012, 134, 10349–10352;
- 2bT. Nishinaga, T. Ohmae, K. Aita, M. Takase, M. Iyoda, T. Arai, Y. Kunugi, Chem. Commun. 2013, 49, 5354–5356;
- 2cJ. Y. Shin, T. Yamada, H. Yoshikawa, K. Awaga, H. Shinokubo, Angew. Chem. Int. Ed. 2014, 53, 3096–3101; Angew. Chem. 2014, 126, 3160–3165.
- 3
- 3aR. Gleiter, G. Haberhauer, Aromaticity and Other Conjugation Effects, Wiley-VCH, Weiheim, 2012;
- 3bA. Minsky, A. Y. Meyer, M. Rabinovitz, Tetrahedron Lett. 1982, 23, 5351–5354;
- 3cA. Minsky, A. Y. Meyer, M. Rabinovitz, Tetrahedron 1985, 41, 785–791.
- 4
- 4aK. Hafner, R. Dönges, E. Goedecke, R. Kaiser, Angew. Chem. Int. Ed. Engl. 1973, 12, 337–339; Angew. Chem. 1973, 85, 362–364;
- 4bR. Dönges, K. Hafner, H. J. Lindner, Tetrahedron Lett. 1976, 17, 1345–1348.
10.1016/S0040-4039(00)78060-9 Google Scholar
- 5K. Brand, Ber. Dtsch. Chem. Ges. 1912, 45, 3071–3077.
- 6C. C. Chuen, S. W. Fenton, J. Org. Chem. 1958, 23, 1538–1539.
- 7
- 7aM. Saito, M. Nakamura, T. Tajima, M. Yoshioka, Angew. Chem. Int. Ed. 2007, 46, 1504–1507; Angew. Chem. 2007, 119, 1526–1529;
- 7bT. Kawase, A. Konishi, Y. Hirao, K. Matsumoto, H. Kurata, T. Kubo, Chem. Eur. J. 2009, 15, 2653–2661;
- 7cH. Zhang, T. Karasawa, H. Yamada, A. Wakamiya, S. Yamaguchi, Org. Lett. 2009, 11, 3076–3079;
- 7dZ. U. Levi, T. D. Tilley, J. Am. Chem. Soc. 2009, 131, 2796–2797;
- 7eA. Konishi, T. Fujiwara, N. Ogawa, Y. Hirao, K. Matsumoto, H. Kurata, T. Kubo, C. Kitamura, T. Kawase, Chem. Lett. 2010, 39, 300–301;
- 7fX. Yin, Y. Lin, Y. Zhu, Y. Kan, Y. Li, D. Zhu, Org. Lett. 2011, 13, 1520–1523;
- 7gA. S. K. Hashmi, M. Wieteck, I. Braun, P. Nösel, L. Jongbloed, M. Rudolph, F. Rominger, Adv. Synth. Catal. 2012, 354, 555–562;
- 7hP. Rivera-Fuentes, M. v. W. Rekowski, W. B. Schweizer, J. P. Gisselbrecht, C. Boudon, F. Diederich, Org. Lett. 2012, 14, 4066–4069;
- 7iC. Chen, M. Harhausen, R. Liedtke, K. Bussmann, A. Fukazawa, S. Yamaguchi, J. L. Petersen, C. G. Daniliuc, R. Fröhlich, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2013, 52, 5992–5996; Angew. Chem. 2013, 125, 6108–6112;
- 7jT. Maekawa, Y. Segawa, K. Itami, Chem. Sci. 2013, 4, 2369–2373;
- 7kJ. Zhao, K. Oniwa, N. Asao, Y. Yamamoto, T. Jin, J. Am. Chem. Soc. 2013, 135, 10222–10225;
- 7lC. Hu, Q. Zhang, Chin. J. Chem. 2013, 31, 1404–1408;
- 7mG. London, M. v. W. Rekowski, O. Dumele, W. B. Schweizer, J. P. Gisselbrecht, C. Boudon, F. Diederich, Chem. Sci. 2014, 5, 965–972;
- 7nH. Li, X. Y. Wang, B. Wei, L. Xu, W. X. Zhang, J. Pei, Z. Xi, Nat. Commun. 2014, 5, 4508–4517;
- 7oJ. Shen, D. Yuan, Y. Qiao, X. Shen, Z. Zhang, Y. Zhong, Y. Yi, X. Zhu, Org. Lett. 2014, 16, 4924–4927;
- 7pK. Takahashi, S. Ito, R. Shintani, K. Nozaki, Chem. Sci. 2017, 8, 101–107.
- 8
- 8aG. Babu, A. Orita, J. Otera, Chem. Lett. 2008, 37, 1296–1297;
- 8bF. Xu, L. Peng, A. Orita, J. Otera, Org. Lett. 2012, 14, 3970–3973.
- 9
- 9aT. Kawase, T. Fujiwara, C. Kitamura, A. Konishi, Y. Hirano, K. Matsumoto, H. Kurata, T. Kubo, S. Shinamura, H. Mori, E. Miyazaki, K. Takimiya, Angew. Chem. Int. Ed. 2010, 49, 7728–7732; Angew. Chem. 2010, 122, 7894–7898;
- 9bM. Nakano, I. Osaka, K. Takimiya, T. Koganezawa, J. Mater. Chem. C 2014, 2, 64–70;
- 9cG. Dai, J. Chang, W. Zhang, S. Bai, K. W. Huang, J. Xu, C. Chi, Chem. Commun. 2015, 51, 503–506;
- 9dG. Dai, J. Chang, X. Shi, W. Zhang, B. Zheng, K. W. Huang, C. Chi, Chem. Eur. J. 2015, 21, 2019–2028;
- 9eC. Liu, S. Xu, W. Zhu, X. Zhu, W. Hu, Z. Li, Z. Wang, Chem. Eur. J. 2015, 21, 17016–17022.
- 10T. C. Walsgrove, F. Sondheimer, Tetrahedron Lett. 1978, 19, 2719–2722.
10.1016/S0040-4039(01)91586-2 Google Scholar
- 11A. J. Boydston, M. M. Haley, R. V. Williams, J. R. Armantrout, J. Org. Chem. 2002, 67, 8812–8819.
- 12T. Nishinaga, H. Nakayama, N. Nodera, K. Komatsu, Tetrahedron Lett. 1998, 39, 7139–7142.
- 13S. Kato, N. Takahashi, H. Tanaka, A. Kobayashi, T. Yoshihara, S. Tobita, T. Yamanobe, H. Uehara, Y. Nakamura, Chem. Eur. J. 2013, 19, 12138–12151.
- 14
- 14aB. S. Young, D. T. Chase, J. L. Marshall, C. L. Vonnegut, L. N. Zakharov, M. M. Haley, Chem. Sci. 2014, 5, 1008–1014;
- 14bJ. L. Marshall, K. Uchida, C. K. Frederickson, C. Schütt, A. M. Zeidell, K. P. Goetz, T. W. Finn, K. Jarolimek, L. N. Zakharov, C. Risko, R. Herges, O. D. Jurchescu, M. M. Haley, Chem. Sci. 2016, 7, 5547–5558. In the course of the submission of this paper, the following paper has been published, where the antiaromaticity of a phenanthrene-fused pentalene has been briefly described:
- 14cC. K. Frederickson, L. N. Zakharov, M. M. Haley, J. Am. Chem. Soc. 2016, 138, 16827–16838.
- 15
- 15aA. Iida, S. Yamaguchi, J. Am. Chem. Soc. 2011, 133, 6952–6955;
- 15bA. Iida, A. Sekioka, S. Yamaguchi, Chem. Sci. 2012, 3, 1461–1466.
- 16
- 16aA. Fukazawa, H. Oshima, Y. Shiota, S. Takahashi, K. Yoshizawa, S. Yamaguchi, J. Am. Chem. Soc. 2013, 135, 1731–1734;
- 16bA. Fukazawa, H. Oshima, S. Shimizu, N. Kobayashi, S. Yamaguchi, J. Am. Chem. Soc. 2014, 136, 8738–8745;
- 16cH. Oshima, A. Fukazawa, T. Sasamori, S. Yamaguchi, Angew. Chem. Int. Ed. 2015, 54, 7636–7639; Angew. Chem. 2015, 127, 7746–7749.
- 17
- 17aB. H. Lipshutz, K. Siegmann, E. Garcia, F. Kayser, J. Am. Chem. Soc. 1993, 115, 9276–9282;
- 17bY. Miyake, M. Wu, M. J. Rahman, M. Iyoda, Chem. Commun. 2005, 411–413;
- 17cY. Miyake, M. Wu, M. J. Rahman, Y. Kuwatani, M. Iyoda, J. Org. Chem. 2006, 71, 6110–6117;
- 17dM. J. Rahman, J. Yamakawa, A. Matsumoto, H. Enozawa, T. Nishinaga, K. Kamada, M. Iyoda, J. Org. Chem. 2008, 73, 5542–5548.
- 18As these compounds were not separable due to their poor solubilities, the obtained mixture was directly used for next transformation. Product yields of 8 a, 10, and 11 were estimated to be 41 %, 5 %, and 4 %, respectively, based on the weight of the mixture and the molar ratio determined by the 1H NMR spectrum.
- 19
- 19aR. B. Woodward, R. Hoffman, J. Am. Chem. Soc. 1965, 87, 395–397;
- 19bR. B. Woodward, R. Hoffman, The Conservation of Orbital Symmetry, Chemie, Weinheim, 1970;
- 19cK. Fukui, Theory of Orientation and Stereoselection, Springer, Berlin, 1970.
10.1007/BFb0051113 Google Scholar
- 20M. Taniguchi, Y. Takeyama, K. Fugami, K. Oshima, K. Utimoto, Bull. Chem. Soc. Jpn. 1991, 64, 2593–2595.
- 21I. Willner, J. Y. Becker, M. Rabinovitz, J. Am. Chem. Soc. 1979, 101, 395–401.
- 22
- 22aJ. Kruszewski, T. M. Krygowski, Tetrahedron Lett. 1972, 13, 3839–3842;
- 22bT. M. Krygowski, J. Chem. Inf. Comput. Sci. 1993, 33, 70–78.
- 23
- 23aP. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, N. J. R. van Eikema Hommes, J. Am. Chem. Soc. 1996, 118, 6317–6318;
- 23bC. Corminboeuf, T. Heine, G. Seifert, P. v. R. Schleyer, J. Weber, Phys. Chem. Chem. Phys. 2004, 6, 273–276;
- 23cH. Fallah-Bagher-Shaidaei, C. S. Wannere, C. Corminboef, R. Puchta, P. v. R. Schleyer, Org. Lett. 2006, 8, 863–866;
- 23dN. S. Mills, K. B. Llagostera, J. Org. Chem. 2007, 72, 9163–9169.
- 24K. Hafner, H. U. Süss, Angew. Chem. Int. Ed. Engl. 1973, 12, 575–577; Angew. Chem. 1973, 85, 626–628.
- 25J. K. Kendall, H. Shechter, J. Org. Chem. 2001, 66, 6643–6649.