Reversible Stereoselective Folding/Unfolding Fueled by the Interplay of Photoisomerism and Hydrogen Bonding
Dr. Christopher R. Opie
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Dr. Naoya Kumagai
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Masakatsu Shibasaki
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorDr. Christopher R. Opie
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Dr. Naoya Kumagai
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Masakatsu Shibasaki
Institute of Microbial Chemistry (BIKAKEN), Tokyo, 3-14-23 Kamiosaki, Shinagawa-ku, Tokyo, 141-0021 Japan
Search for more papers by this authorGraphical Abstract
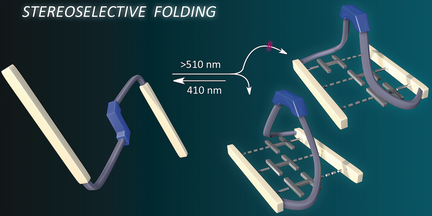
Know when to fold 'em: A linear molecular architecture equipped with complementary three-fold hydrogen bonding units embedded with a photoswitchable trans-tetrafluoroazobenzene moiety was synthesized. The trans to cis photoisomerism of the azobenzene unit induced changes in the molecular architecture as a result of intramolecular hydrogen bonding as evidenced by NMR spectroscopy and size exclusion chromatography.
Abstract
A linear molecular architecture equipped with complementary three-fold hydrogen-bonding units embedded with a photoswitchable trans-tetrafluoroazobenzene moiety was synthesized. The transto cis photoisomerism of the azobenzene unit induced drastic changes in the molecular architecture as a result of intramolecular hydrogen bonding as evidenced by NMR spectroscopy and size exclusion chromatography. A minute stereogenic element in the linear trans state enabled stereoselective folding into the cis state, thus producing a globular architecture with enhanced chiroptical property.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201610279-sup-0001-misc_information.pdf22.3 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. Hecht, I. Huc, Foldamers: structure, properties, and applications, Wiley-VCH, Weinheim, 2007.
10.1002/9783527611478 Google Scholar
- 2For reviews, see:
- 2aD. J. Hill, M. J. Mio, R. B. Prince, T. S. Hughes, J. S. Moore, Chem. Rev. 2001, 101, 3893;
- 2bD.-W. Zhang, X. Zhao, Z.-T. Li, Acc. Chem. Res. 2014, 47, 1961;
- 2cM. Barboiu, A. M. Stadler, J.-M. Lehn, Angew. Chem. Int. Ed. 2016, 55, 4130; Angew. Chem. 2016, 128, 4200;
- 2dB. A. Le Bailly, J. Clayden, Chem. Commun. 2016, 52, 4852.
- 3
- 3aG. Lautrette, C. Aube, Y. Ferrand, M. Pipelier, V. Blot, C. Thobie, B. Kauffmann, D. Dubreuil, I. Huc, Chem. Eur. J. 2014, 20, 1547;
- 3bS. Lee, Y. Hua, A. H. Flood, J. Org. Chem. 2014, 79, 8383;
- 3cM. Guentner, E. Uhl, P. Mayer, H. Dube, Chem. Eur. J. 2016, 22, 16433.
- 4
- 4aC. Dolain, V. Maurizot, I. Huc, Angew. Chem. Int. Ed. 2003, 42, 2738; Angew. Chem. 2003, 115, 2844;
- 4bE. Kolomiets, V. Berl, I. Odriozola, A.-M. Stadler, N. Kyritsakas, J.-M. Lehn, Chem. Commun. 2003, 2868;
- 4cH.-Y. Hu, J.-F. Xiang, Y. Yang, C.-F. Chen, Org. Lett. 2008, 10, 1275;
- 4dW. Zhao, Y. Wang, J. Shang, Y. Che, H. Jiang, Chem. Eur. J. 2015, 21, 7731.
- 5V. R. Naidu, J.-M. Suk, G.-W. Lee, K.-S. Jeong, Bull. Korean Chem. Soc. 2009, 30, 482.
- 6
- 6aR. B. Prince, T. Okada, J. S. Moore, Angew. Chem. Int. Ed. 1999, 38, 233;
10.1002/(SICI)1521-3773(19990115)38:1/2<233::AID-ANIE233>3.0.CO;2-Y CAS Web of Science® Google ScholarAngew. Chem. 1999, 111, 245;
- 6bM. Barboiu, J.-M. Lehn, Proc. Natl. Acad. Sci. USA 2002, 99, 5201;
- 6cN. Sakamoto, C. Ikeda, M. Yamamura, T. Nabeshima, Chem. Commun. 2012, 48, 4818.
- 7H. Okuda, Y. Koyama, S. Uchida, T. Michinobu, H. Sogawa, T. Takata, ACS Macro Lett. 2015, 4, 462.
- 8
- 8aS.-L. Dong, M. Löweneck, T. E. Schrader, W. J. Schreier, W. Zinth, L. Moroder, C. Renner, Chem. Eur. J. 2006, 12, 1114;
- 8bA. Khan, C. Kaiser, S. Hecht, Angew. Chem. Int. Ed. 2006, 45, 1878; Angew. Chem. 2006, 118, 1912;
- 8cG. Haberhauer, C. Kallweit, Angew. Chem. Int. Ed. 2010, 49, 2418; Angew. Chem. 2010, 122, 2468;
- 8dY. Hua, A. H. Flood, J. Am. Chem. Soc. 2010, 132, 12838;
- 8eY. Wang, F. Bie, H. Jiang, Org. Lett. 2010, 12, 3630;
- 8fZ. Yu, S. Hecht, Angew. Chem. Int. Ed. 2011, 50, 1640; Angew. Chem. 2011, 123, 1678;
- 8gS. Braun, M. Bockmann, D. Marx, ChemPhysChem 2012, 13, 1440.
- 9For reviews, see:
- 9aH. Dürr, H. Bouas-Laurent, Photochromism : molecules and systems, Rev. ed., Elsevier, Amsterdam, Boston, 2003;
- 9bS. Hecht, Small 2005, 1, 26;
- 9cY. Zhao, T. Ikeda, Smart Light-responsive Materials: Azobenzene-containing Polymers and Liquid Crystals, Wiley, Hoboken, 2009;
10.1002/9780470439098 Google Scholar
- 9dM. M. Russew, S. Hecht, Adv. Mater. 2010, 22, 3348;
- 9eM. Irie, T. Seki, Y. Yokoyama, New Frontiers in Photochromism, Springer, London, 2013.
10.1007/978-4-431-54291-9 Google Scholar
- 10For a review, see: W. Szymański, J. M. Beierle, H. A. Kistemaker, W. A. Velema, B. L. Feringa, Chem. Rev. 2013, 113, 6114.
- 11D. Zhao, T. van Leeuwen, J. Cheng, B. L. Feringa, Nat. Chem. DOI: 10.1038/NCHEM2668.
- 12
- 12aR. A. Brown, V. Diemer, S. J. Webb, J. Clayden, Nat. Chem. 2013, 5, 853;
- 12bJ. Brioche, S. J. Pike, S. Tshepelevitsh, I. Leito, G. A. Morris, S. J. Webb, J. Clayden, J. Am. Chem. Soc. 2015, 137, 6680;
- 12cR. Wechsel, J. Raftery, D. Cavagnat, G. Guichard, J. Clayden, Angew. Chem. Int. Ed. 2016, 55, 9657; Angew. Chem. 2016, 128, 9809.
- 13
- 13aD. H. Appella, L. A. Christianson, I. L. Karle, D. R. Powell, S. H. Gellman, J. Am. Chem. Soc. 1996, 118, 13071;
- 13bD. Seebach, M. Overhand, F. N. M. Kiihnle, A. B. Martinoni, Helv. Chim. Acta 1996, 79, 913;
- 13cD. Seebach, J. L. Matthews, Chem. Commun. 1997, 2015;
- 13dS. H. Gellman, Acc. Chem. Res. 1998, 31, 173;
- 13eI. M. Mándity, F. Fülöp, Expert Opin. Drug Discovery 2015, 10, 1163.
- 14For a review, see: S. K. Yang, S. C. Zimmerman, Isr. J. Chem. 2013, 53, 511.
- 15
- 15aT. J. Murray, S. C. Zimmerman, J. Am. Chem. Soc. 1992, 114, 4010;
- 15bS. V. Kolotuchin, S. C. Zimmerman, J. Am. Chem. Soc. 1998, 120, 9092;
- 15cM. Mascal, N. M. Hext, R. Warmuth, J. R. Arnall-Culliford, M. H. Moore, J. P. Turkenburg, J. Org. Chem. 1999, 64, 8479;
- 15dP. S. Corbin, S. C. Zimmerman, P. A. Thiessen, N. A. Hawryluk, T. J. Murray, J. Am. Chem. Soc. 2001, 123, 10475.
- 16L. V. Sudha, D. N. Sathyanarayana, J. Mol. Struct. 1984, 125, 89.
- 17
- 17aD. Bléger, J. Schwarz, A. M. Brouwer, S. Hecht, J. Am. Chem. Soc. 2012, 134, 20597;
- 17bC. Knie, M. Utecht, F. Zhao, H. Kulla, S. Kovalenko, A. M. Brouwer, P. Saalfrank, S. Hecht, D. Bleger, Chem. Eur. J. 2014, 20, 16492;
- 17cJ. Moreno, M. Gerecke, L. Grubert, S. A. Kovalenko, S. Hecht, Angew. Chem. Int. Ed. 2016, 55, 1544; Angew. Chem. 2016, 128, 1569.
- 18
- 18aO. S. Bushuyev, A. Tomberg, T. Friščić, C. J. Barrett, J. Am. Chem. Soc. 2013, 135, 12556;
- 18bS. Castellanos, A. Goulet-Hanssens, F. Zhao, A. Dikhtiarenko, A. Pustovarenko, S. Hecht, J. Gascon, F. Kapteijn, D. Bleger, Chem. Eur. J. 2016, 22, 746;
- 18cQ. Liu, H. Dong, Y. Li, H. Li, D. Chen, L. Wang, Q. Xu, J. Lu, Chem. Asian J. 2016, 11, 512.
- 19R. Chinchilla, C. Najera, Chem. Soc. Rev. 2011, 40, 5084.
- 20Synthesis of 1 a–f is detailed in the Supporting Information.
- 21While the irradiation rate to cis appeared to be largely independent of concentration, irradiation to trans was not. Interestingly, while irradiation with λ=400 nm gave faster conversion to trans at 10 mm, the PSS was lower for this wavelength. The highest photoconversion was achieved with λ=410 nm (87 %, 0.2 mm). At this concentration, irradiation with λ=400 nm gave 80 %.
- 22See the Supporting Information.
- 23For further structural characterization of trans-1 a, see the Supporting Information (Section 5.1).
- 24Chemical shifts of Hurea1 of trans-1 a and cis-1 a in the 1H NMR spectra were different in other solvents (see the Supporting Information). The mode of the hydrogen-bonding interaction of Hurea1 for trans (closed pyridylurea) and cis (hydrogen bonding with the naphthyridine) isomers was likely different based on NOESY analysis.
- 25Structural optimization (B3LYP/6-31G) of potential pseudo-diastereomers of folded cis-1 c (Figure 3 b) revealed that (S)-Me-M-cis-1 c was 3.2 kcal mol−1 more favored than (S)-Me-P-cis-1 c, thus supporting the experimental results. MD simulations of (S)-Me-M-cis-1 c suggested that the hydrogen-bonded folded conformation is likely to be stable. See the Supporting Information for details.
- 26J. Solà, G. A. Morris, J. Clayden, J. Am. Chem. Soc. 2011, 133, 3712, See also ref. [2d].
- 27DFT calculation of folded cis-1 d-f indicated that the (S)-Me-M-cis pseudo-diastereomers were consistently favored over the corresponding (S)-Me-P-cis pseudodiastereomers. See the Supporting Information for details and MD simulations.