Template-Assisted meta-C−H Alkylation and Alkenylation of Arenes
Sukdev Bag
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai-, 400 076 India
Search for more papers by this authorDr. Ramasamy Jayarajan
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai-, 400 076 India
Department of Biosciences and Bioengineering, Indian Institute of Technology Bombay, India
Search for more papers by this authorRahul Mondal
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai-, 400 076 India
Search for more papers by this authorCorresponding Author
Prof. Dr. Debabrata Maiti
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai-, 400 076 India
Search for more papers by this authorSukdev Bag
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai-, 400 076 India
Search for more papers by this authorDr. Ramasamy Jayarajan
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai-, 400 076 India
Department of Biosciences and Bioengineering, Indian Institute of Technology Bombay, India
Search for more papers by this authorRahul Mondal
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai-, 400 076 India
Search for more papers by this authorCorresponding Author
Prof. Dr. Debabrata Maiti
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai-, 400 076 India
Search for more papers by this authorGraphical Abstract
Abstract
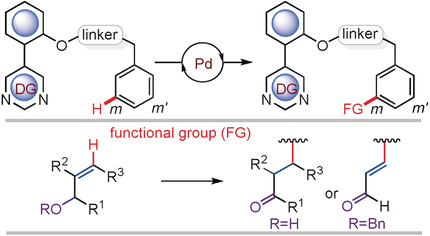
To expand the scope of meta-functionalization, a pyrimidine-based template effective for the formation of β-aryl aldehydes and ketones, using allyl alcohols, by meta-C−H activation of benzylsulfonyl esters is described. In addition, α,β-unsaturated aldehydes were generated by in situ olefination and deprotection of allyl benzyl ethers. These new functionalizations at the meta-position of an arene have also been successfully implemented in benzylphosphonate, phenethyl carbonyl, and phenethylsulfonyl ester scaffolds. Key to these successful new functionalizations is the creation of an electropositive palladium center by accepting the electron cloud from the metal to the energetically low-lying π-orbitals of pyrimidine ring, and it favors coordination of allyl alcohol to the metal center.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201611360-sup-0001-misc_information.pdf9.4 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews on C−H activation, see:
- 1aL. Ackermann, R. Vicente, A. R. Kapdi, Angew. Chem. Int. Ed. 2009, 48, 9792; Angew. Chem. 2009, 121, 9976;
- 1bX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. Int. Ed. 2009, 48, 5094; Angew. Chem. 2009, 121, 5196;
- 1cC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215;
- 1dN. Kuhl, M. N. Hopkinson, J. Wencel-Delord, F. Glorius, Angew. Chem. Int. Ed. 2012, 51, 10236; Angew. Chem. 2012, 124, 10382;
- 1eM. S. Sigman, E. W. Werner, Acc. Chem. Res. 2012, 45, 874;
- 1fL. Ackermann, Acc. Chem. Res. 2014, 47, 281;
- 1gS. De Sarkar, W. Liu, S. I. Kozhushkov, L. Ackermann, Adv. Synth. Catal. 2014, 356, 1461;
- 1hJ. Li, S. De Sarkar, L. Ackermann, Top. Organomet. Chem. 2016, 55, 217.
- 2For other approaches towards meta-C−H functionalization, see:
- 2aJ. M. Murphy, X. Liao, J. F. Hartwig, J. Am. Chem. Soc. 2007, 129, 15434;
- 2bR. J. Phipps, M. J. Gaunt, Science 2009, 323, 1593;
- 2cY.-H. Zhang, B.-F. Shi, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 5072;
- 2dO. Saidi, J. Marafie, A. E. W. Ledger, P. M. Liu, M. F. Mahon, G. Kociok-Köhn, M. K. Whittlesey, C. G. Frost, J. Am. Chem. Soc. 2011, 133, 19298;
- 2eJ. Cornella, M. Righi, I. Larrosa, Angew. Chem. Int. Ed. 2011, 50, 9429; Angew. Chem. 2011, 123, 9601;
- 2fH. A. Duong, R. E. Gilligan, M. L. Cooke, R. J. Phipps, M. J. Gaunt, Angew. Chem. Int. Ed. 2011, 50, 463; Angew. Chem. 2011, 123, 483;
- 2gN. Hofmann, L. Ackermann, J. Am. Chem. Soc. 2013, 135, 5877;
- 2hJ. Luo, S. Preciado, I. Larrosa, J. Am. Chem. Soc. 2014, 136, 4109;
- 2iM. Tobisu, N. Chatani, Science 2014, 343, 850;
- 2jA. J. Martínez-Martínez, A. R. Kennedy, R. E. Mulvey, C. T. O'Hara, Science 2014, 346, 834;
- 2kJ. Schranck, A. Tlili, M. Beller, Angew. Chem. Int. Ed. 2014, 53, 9426; Angew. Chem. 2014, 126, 9580;
- 2lX.-C. Wang, W. Gong, L.-Z. Fang, R.-Y. Zhu, S. Li, K. M. Engle, J.-Q. Yu, Nature 2015, 519, 334;
- 2mP.-X. Shen, X.-C. Wang, P. Wang, R.-Y. Zhu, J.-Q. Yu, J. Am. Chem. Soc. 2015, 137, 11574;
- 2nZ. Dong, J. Wang, G. Dong, J. Am. Chem. Soc. 2015, 137, 5887;
- 2oJ. Luo, S. Preciado, I. Larrosa, Chem. Commun. 2015, 51, 3127;
- 2pC. J. Teskey, A. Y. W. Lui, M. F. Greaney, Angew. Chem. Int. Ed. 2015, 54, 11677; Angew. Chem. 2015, 127, 11843;
- 2qA. J. Paterson, S. St John-Campbell, M. F. Mahon, N. J. Press, C. G. Frost, Chem. Commun. 2015, 51, 12807;
- 2rY. Kuninobu, H. Ida, M. Nishi, M. Kanai, Nat. Chem. 2015, 7, 712;
- 2sL. Ackermann, J. Li, Nat. Chem. 2015, 7, 686;
- 2tJ. Li, S. Warratz, D. Zell, S. De Sarkar, E. E. Ishikawa, L. Ackermann, J. Am. Chem. Soc. 2015, 137, 13894;
- 2uJ. Luo, S. Preciado, S. O. Araromi, I. Larrosa, Chem. Asian J. 2015, 6, 5595;
- 2vN. Y. P. Kumar, A. Bechtoldt, K. Raghuvanshi, L. Ackermann, Angew. Chem. Int. Ed. 2016, 55, 6929; Angew. Chem. 2016, 128, 7043;
- 2wR. Bisht, B. Chattopadhyay, J. Am. Chem. Soc. 2016, 138, 84;
- 2xZ. Fan, J. Ni, A. Zhang, J. Am. Chem. Soc. 2016, 138, 8470;
- 2yP. Wang, M. E. Farmer, X. Huo, P. Jain, P.-X. Shen, M. Ishoey, J. E. Bradner, S. R. Wisniewski, M. E. Eastgate, J.-Q. Yu, J. Am. Chem. Soc. 2016, 138, 9269;
- 2zP. Wang, G.-C. Li, P. Jain, M. E. Farmer, J. He, P.-X. Shen, J.-Q. Yu, J. Am. Chem. Soc. 2016, 138, 14092.
- 3For examples of meta-C−H activation by chelation assistance, see:
- 3aD. Leow, G. Li, T.-S. Mei, J.-Q. Yu, Nature 2012, 486, 518;
- 3bH.-X. Dai, G. Li, X.-G. Zhang, A. F. Stepan, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 7567;
- 3cL. Wan, N. Dastbaravardeh, G. Li, J.-Q. Yu, J. Am. Chem. Soc. 2013, 135, 18056;
- 3dS. Lee, H. Lee, K. L. Tan, J. Am. Chem. Soc. 2013, 135, 18778;
- 3eR.-Y. Tang, G. Li, J.-Q. Yu, Nature 2014, 507, 215;
- 3fY.-F. Yang, G.-J. Cheng, P. Liu, D. Leow, T.-Y. Sun, P. Chen, X. Zhang, J.-Q. Yu, Y.-D. Wu, K. N. Houk, J. Am. Chem. Soc. 2014, 136, 344;
- 3gG. Yang, P. Lindovska, D. Zhu, J. Kim, P. Wang, R.-Y. Tang, M. Movassaghi, J.-Q. Yu, J. Am. Chem. Soc. 2014, 136, 10807;
- 3hM. Bera, A. Modak, T. Patra, A. Maji, D. Maiti, Org. Lett. 2014, 16, 5760;
- 3iY. Deng, J.-Q. Yu, Angew. Chem. Int. Ed. 2015, 54, 888; Angew. Chem. 2015, 127, 902;
- 3jM. Bera, A. Maji, S. K. Sahoo, D. Maiti, Angew. Chem. Int. Ed. 2015, 54, 8515; Angew. Chem. 2015, 127, 8635;
- 3kS. Li, H. Ji, L. Cai, G. Li, Chem. Sci. 2015, 6, 5595;
- 3lM. Bera, A. Maji, S. K. Sahoo, D. Maiti, ACS Catal. 2016, 6, 3575;
- 3mT. Patra, R. Watile, S. Agasti, N. Togati, D. Maiti, Chem. Commun. 2016, 52, 2027;
- 3nA. Maji, B. Bhaskararao, S. Singha, R. B. Sunoj, D. Maiti, Chem. Sci. 2016, 7, 3147;
- 3oS. Li, L. Cai, H. Ji, L. Yang, G. Li, Nat. Commun. 2016, 7, 10443.
- 4For selected examples of Heck-type reactions, see:
- 4aR. F. Heck, in Comprehensive Organic Synthesis, Vol. 4 (Ed.: ), Pergamon, New York, 1991, Ch. 4.3, ;
- 4bI. P. Beletskaya, A. V. Cheprakov, Chem. Rev. 2000, 100, 3009;
- 4cA. B. Dounay, L. E. Overman, Chem. Rev. 2003, 103, 2945;
- 4dJ. Muzart, Tetrahedron 2005, 61, 4179;
- 4eE. W. Werner, M. S. Sigman, J. Am. Chem. Soc. 2011, 133, 9692.
- 5
- 5aB. J. Stokes, S. M. Opra, M. S. Sigman, J. Am. Chem. Soc. 2012, 134, 11408;
- 5bE. W. Werner, T.-S. Mei, A. J. Burckle, M. S. Sigman, Science 2012, 338, 1455;
- 5cH. Renata, Q. Zhou, P. S. Baran, Science 2013, 339, 59;
- 5dT.-S. Mei, E. W. Werner, A. J. Burckle, M. S. Sigman, J. Am. Chem. Soc. 2013, 135, 6830;
- 5eT.-S. Mei, H. H. Patel, M. S. Sigman, Nature 2014, 508, 340;
- 5fE. Larionov, L. Lin, L. Guénée, C. Mazet, J. Am. Chem. Soc. 2014, 136, 16882;
- 5gH. H. Patel, M. S. Sigman, J. Am. Chem. Soc. 2015, 137, 3462;
- 5hH. Li, C. Mazet, J. Am. Chem. Soc. 2015, 137, 10720;
- 5iL. Lin, C. Romano, C. Mazet, J. Am. Chem. Soc. 2016, 138, 10344.
- 6For selected examples of β-aryl aldehyde and keto from allyl alcohol, see:
- 6aL. Huang, Q. Wang, J. Qi, X. Wu, K. Huang, H. Jiang, Chem. Sci. 2013, 4, 2665;
- 6bZ. Shi, M. B. Arapinis, F. Glorius, Chem. Commun. 2013, 49, 6489;
- 6cD. Kalsi, R. A. Laskar, N. Barsu, J. R. Premkumar, B. Sundararaju, Org. Lett. 2016, 18, 4198.
- 7See the Supporting Information for a detailed description.
- 8Pyridine-based DG for meta-C−H functionalization: L. Chu, M. Shang, K. Tanaka, Q. Chen, N. Pissarnitski, E. Streckfuss, J.-Q. Yu, ACS Cent. Sci. 2015, 1, 394.
- 9
- 9a“σ-Pyridine Coordination Compounds with Transition Metals”: Chemistry of Heterocyclic Compounds: Pyridine Metal Complexes, Part 6, Vol. 14, (Eds.: ), Wiley, Hoboken, Ch. 3, 1985;
- 9b“π-Coordination Compounds of Pyridines with Metals”: Chemistry of Heterocyclic Compounds: Pyridine Metal Complexes, Part 6, Vol. 14 (Eds.: ), Wiley, Hoboken, 1985, Ch. 5.
- 10D. Chen, S.-J. Su, Y. Cao, J. Mater. Chem. C 2014, 2, 9565.
- 11
- 11aM. O. Ratnikov, V. V. Tumanov, W. A. Smit, Angew. Chem. Int. Ed. 2008, 47, 9739; Angew. Chem. 2008, 120, 9885;
- 11bJ. Wencel-Delord, F. Colobert, Org. Chem. Front. 2016, 3, 394;
- 11cH. Wu, Y. Wan, W. Wang, Y. Wang, N. Zhou, W. Zhang, X. Li, Z. Zhang, X. Zhu, Polym. Chem. 2015, 6, 2620.
- 12
- 12aG. Sabitha, S. Nayak, M. Bhikshapathi, J. S. Yadav, Org. Lett. 2011, 13, 382;
- 12bP. Mamone, M. F. Grunberg, A. Fromm, B. A. Khan, L. J. Gooßen, Org. Lett. 2012, 14, 3716.