Enantioselective Dearomatization of Naphthol Derivatives with Allylic Alcohols by Cooperative Iridium and Brønsted Acid Catalysis
Dan Shen
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou, 310036 China
Search for more papers by this authorQiliang Chen
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou, 310036 China
Search for more papers by this authorPeipei Yan
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou, 310036 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaofei Zeng
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou, 310036 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guofu Zhong
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou, 310036 China
Search for more papers by this authorDan Shen
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou, 310036 China
Search for more papers by this authorQiliang Chen
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou, 310036 China
Search for more papers by this authorPeipei Yan
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou, 310036 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Xiaofei Zeng
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou, 310036 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Guofu Zhong
College of Materials, Chemistry and Chemical Engineering, Hangzhou Normal University, Hangzhou, 310036 China
Search for more papers by this authorGraphical Abstract
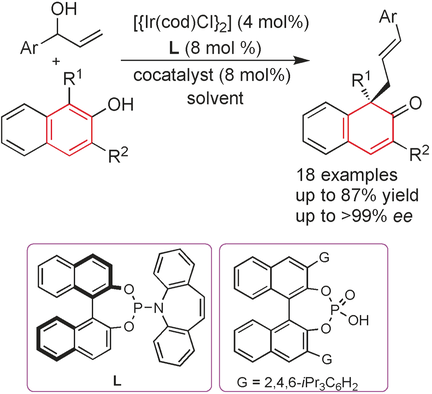
Co-op: The cooperative dual-catalytic system of a chiral iridium complex and phosphoric acid achieves highly enantioselective allylic dearomatization reactions of naphthols with racemic secondary allylic alcohols. The desired β-naphthalenones, bearing an all-carbon quaternary center, were obtained in good yields. Both β-naphthalenone enantiomers can be obtained by simply employing opposite enantiomeric ligands.
Abstract
The combination of a transition-metal catalyst and organocatalyst was designed to achieve a highly enantioselective system for the allylic dearomatization reaction of naphthols with racemic secondary allylic alcohols. The desired β-naphthalenones, bearing an all-carbon quaternary center, were obtained in good yields with high chemo- and enantioselectivities. The cooperative catalytic system, involving a chiral iridium complex and phosphoric acid, provided measurable improvements in yields, and chemo- and enantioselectivities relative to single-catalyst systems. Control experiments indicated that the chiral iridium complex functions as a key species in the control of the absolute configuration, thus enabling the formation of both β-naphthalenone enantiomers by simply employing opposite enantiomeric ligands.
References
- 1For selected recent reviews on dearomatization reactions, see:
- 1aW.-T. Wu, L. Zhang, S.-L. You, Chem. Soc. Rev. 2016, 45, 1570;
- 1bC.-X. Zhuo, C. Zheng, S.-L. You, Acc. Chem. Res. 2014, 47, 2558;
- 1cC.-X. Zhuo, W. Zhang, S.-L. You, Angew. Chem. Int. Ed. 2012, 51, 12662; Angew. Chem. 2012, 124, 12834;
- 1dS. P. Roche, J. A. Porco, Jr., Angew. Chem. Int. Ed. 2011, 50, 4068; Angew. Chem. 2011, 123, 4154;
- 1eF. López Ortiz, M. J. Iglesias, I. Fernández, C. M. Andúljar-Sánchez, G. Ruiz-Gómez, Chem. Rev. 2007, 107, 1580.
- 2For selected recent examples, see:
- 2aJ. H. Boyce, J. A. Porco Jr., Angew. Chem. Int. Ed. 2014, 53, 7832; Angew. Chem. 2014, 126, 7966;
- 2bQ. Yang, J. T. Njardarson, C. Draghici, F. Li, Angew. Chem. Int. Ed. 2013, 52, 8648; Angew. Chem. 2013, 125, 8810;
- 2cY. Kobayakawa, M. Nakada, Angew. Chem. Int. Ed. 2013, 52, 7569; Angew. Chem. 2013, 125, 7717;
- 2dQ. Gu, S.-L. You, Chem. Sci. 2011, 2, 1519;
- 2eJ. C. Green, T. R. R. Pettus, J. Am. Chem. Soc. 2011, 133, 1603;
- 2fS. A. Snyder, T. C. Sherwood, A. G. Ross, Angew. Chem. Int. Ed. 2010, 49, 5146; Angew. Chem. 2010, 122, 5272;
- 2gQ. Gu, Z.-Q. Rong, C. Zheng, S.-L. You, J. Am. Chem. Soc. 2010, 132, 4056;
- 2hS. Dong, E. Hamel, R. Bai, D. G. Covell, J. A. Beutler, J. A. Porco Jr., Angew. Chem. Int. Ed. 2009, 48, 1494; Angew. Chem. 2009, 121, 1522;
- 2iS. P. Cook, A. Polara, S. J. Danishefsky, J. Am. Chem. Soc. 2006, 128, 16440;
- 2jK. C. Nicolaou, X. Hao, M. Wartmann, Angew. Chem. Int. Ed. 2005, 44, 756; Angew. Chem. 2005, 117, 766.
- 3
- 3aT. Dohi, N. Takenaga, T. Nakae, Y. Toyoda, M. Yamasaki, M. Shiro, H. Fujioka, A. Maruyama, Y. Kita, J. Am. Chem. Soc. 2013, 135, 4558;
- 3bT. Oguma, T. Katsuki, J. Am. Chem. Soc. 2012, 134, 20017;
- 3cA. Rudolph, P. H. Bos, A. Meetsma, A. J. Minnaard, B. L. Feringa, Angew. Chem. Int. Ed. 2011, 50, 5834; Angew. Chem. 2011, 123, 5956;
- 3dM. Uyanik, T. Yasui, K. Ishihara, Angew. Chem. Int. Ed. 2010, 49, 2175; Angew. Chem. 2010, 122, 2221;
- 3eS. Quideau, G. Lyvinec, M. Marguerit, K. Bathany, A. Ozanne-Beaudenon, T. Buffeteau, D. Cavagnat, A. Chénedé, Angew. Chem. Int. Ed. 2009, 48, 4605; Angew. Chem. 2009, 121, 4675;
- 3fT. Dohi, A. Maruyama, N. Takenaga, K. Senami, Y. Minamitsuji, H. Fujioka, S. B. Caemmerer, Y. Kita, Angew. Chem. Int. Ed. 2008, 47, 3787; Angew. Chem. 2008, 120, 3847;
- 3gS. Dong, J. Zhu, J. A. Porco, Jr., J. Am. Chem. Soc. 2008, 130, 2738;
- 3hJ. Zhu, N. P. Grigoriadis, J. P. Lee, J. A. Porco, Jr., J. Am. Chem. Soc. 2005, 127, 9342.
- 4S.-G. Wang, X.-J. Liu, Q.-C. Zhao, C. Zheng, S.-B. Wang, S.-L. You, Angew. Chem. Int. Ed. 2015, 54, 14929; Angew. Chem. 2015, 127, 15142.
- 5
- 5aS.-G. Wang, Q. Yin, C.-X. Zhuo, S.-L. You, Angew. Chem. Int. Ed. 2015, 54, 647; Angew. Chem. 2015, 127, 657;
- 5bJ. Nan, J. Liu, H. Zheng, Z. Zuo, L. Hou, H. Hu, Y. Wang, C.-X. Zhuo, X. Luan, Angew. Chem. Int. Ed. 2015, 54, 2356; Angew. Chem. 2015, 127, 2386;
- 5cX. Lian, L. Lin, G. Wang, X. Liu, X. Feng, Chem. Eur. J. 2015, 21, 17453.
- 6
- 6aD. Yang, L. Wang, M. Kai, D. Li, X. Yao, R. Wang, Angew. Chem. Int. Ed. 2015, 54, 9523; Angew. Chem. 2015, 127, 9659;
- 6bD. Yang, L. Wang, F. Han, D. Li, D. Zhao, R. Wang, Angew. Chem. Int. Ed. 2015, 54, 2185; Angew. Chem. 2015, 127, 2213.
- 7
- 7aR. J. Phipps, F. D. Toste, J. Am. Chem. Soc. 2013, 135, 1268;
- 7bQ. Yin, S.-G. Wang, X.-W. Liang, D.-W. Gao, J. Zheng, S.-L. You, Chem. Sci. 2015, 6, 4179.
- 8L. Wang, D. Yang, D. Li, P. Wang, K. Wang, J. Wang, X. Jiang, R. Wang, Chem. Eur. J. 2016, 22, 8483.
- 9For the first asymmetric allylic alkylation, see:
- 9aB. M. Trost, T. J. Fullerton, J. Am. Chem. Soc. 1973, 95, 292; for reviews, see:
- 9bB. M. Trost, D. L. Van Vranken, Chem. Rev. 1996, 96, 395.
- 10For reviews of O-allylation of phenols, see:
- 10aB. M. Trost, M. L. Crawley, Chem. Rev. 2003, 103, 2921;
- 10bZ. Lu, S.-M. Ma, Angew. Chem. Int. Ed. 2008, 47, 258; Angew. Chem. 2008, 120, 264;
- 10cB. M. Trost, T. Zhang, J. D. Sieber, Chem. Sci. 2010, 1, 427.
- 11
- 11aA. V. Malkov, S. L. Davis, I. R. Baxendale, W. L. Mitchell, P. Kočovský, J. Org. Chem. 1999, 64, 2751;
- 11bI. Fernández, R. Hermatschweiler, F. Breher, P. S. Pregosin, L. F. Veiros, M. J. Calhorda, Angew. Chem. Int. Ed. 2006, 45, 6386; Angew. Chem. 2006, 118, 6535;
- 11cY. Yamamoto, K. Itonaga, Org. Lett. 2009, 11, 717;
- 11dY. Suzuki, T. Nemoto, K. Kakugawa, A. Hamajima, Y. Hamada, Org. Lett. 2012, 14, 2350;
- 11eQ.-L. Xu, L.-X. Dai, S.-L. You, Org. Lett. 2012, 14, 2579.
- 12
- 12aT. Nemoto, Y. Ishige, M. Yoshida, Y. Kohno, M. Kanematsu, Y. Hamada, Org. Lett. 2010, 12, 5020;
- 12bM. Yoshida, T. Nemoto, Z. Zhao, Y. Ishige, Y. Hamada, Tetrahedron: Asymmetry 2012, 23, 859;
- 12cQ.-F. Wu, W.-B. Liu, C.-X. Zhuo, Z.-Q. Rong, K.-Y. Ye, S.-L. You, Angew. Chem. Int. Ed. 2011, 50, 4455; Angew. Chem. 2011, 123, 4547;
- 12dC.-X. Zhuo, S.-L. You, Adv. Synth. Catal. 2014, 356, 2020;
- 12eC.-X. Zhuo, S.-L. You, Angew. Chem. Int. Ed. 2013, 52, 10056; Angew. Chem. 2013, 125, 10240.
- 13For reviews of allylic alcohols, see:
- 13aM. Bandini, Angew. Chem. Int. Ed. 2011, 50, 994; Angew. Chem. 2011, 123, 1026;
- 13bB. Sundararaju, M. Achard, C. Bruneau, Chem. Soc. Rev. 2012, 41, 4467;
- 13cN. A. Butt, W.-B. Zhang, Chem. Soc. Rev. 2015, 44, 7929; for recent examples, see:
- 13dM. Raducan, R. Alam, K. J. Szabó, Angew. Chem. Int. Ed. 2012, 51, 13050; Angew. Chem. 2012, 124, 13227;
- 13eJ. M. Larsson, K. J. Szabó, J. Am. Chem. Soc. 2013, 135, 443.
- 14M. Bandini, G. Cera, M. Chiarucci, Synthesis 2012, 504.
- 15
- 15aT. Sandmeier, S. Krautwald, H. F. Zipfel, E. M. Carreira, Angew. Chem. Int. Ed. 2015, 54, 14363; Angew. Chem. 2015, 127, 14571;
- 15bJ. Y. Hamilton, N. Hauser, D. Sarlah, E. M. Carreira, Angew. Chem. Int. Ed. 2014, 53, 10759; Angew. Chem. 2014, 126, 10935;
- 15cS. Krautwald, M. A. Schafroth, D. Sarlah, E. M. Carreira, J. Am. Chem. Soc. 2014, 136, 3020;
- 15dJ. Y. Hamilton, D. Sarlah, E. M. Carreira, J. Am. Chem. Soc. 2014, 136, 3006;
- 15eS. Krautwald, D. Sarlah, M. A. Schafroth, E. M. Carreira, Science 2013, 340, 1065;
- 15fJ. Y. Hamilton, D. Sarlah, E. M. Carreira, Angew. Chem. Int. Ed. 2013, 52, 7532; Angew. Chem. 2013, 125, 7680;
- 15gJ. Y. Hamilton, D. Sarlah, E. M. Carreira, J. Am. Chem. Soc. 2013, 135, 994;
- 15hM. Roggen, E. M. Carreira, Angew. Chem. Int. Ed. 2012, 51, 8652; Angew. Chem. 2012, 124, 8780;
- 15iM. Lafrance, M. Roggen, E. M. Carreira, Angew. Chem. Int. Ed. 2012, 51, 3470; Angew. Chem. 2012, 124, 3527;
- 15jM. Roggen, E. M. Carreira, J. Am. Chem. Soc. 2010, 132, 11917;
- 15kY. Kita, R. D. Kavthe, H. Oda, K. Mashima, Angew. Chem. Int. Ed. 2016, 55, 1098; Angew. Chem. 2016, 128, 1110;
- 15lX. Liang, K. Wei, Y.-R. Yang, Chem. Commun. 2015, 51, 17471.
- 16
- 16aM. Lu, D. Zhu, Y.-P. Lu, X.-F. Zeng, B. Tan, Z.-J. Xu, G. Zhong, J. Am. Chem. Soc. 2009, 131, 4562;
- 16bM. Lu, Y.-P. Lu, D. Zhu, X.-F. Zeng, X.-S. Li, G. Zhong, Angew. Chem. Int. Ed. 2010, 49, 8588; Angew. Chem. 2010, 122, 8770.
- 17Z.-L. Tao, W.-Q. Zhang, D.-F. Chen, A. Adele, L.-Z. Gong, J. Am. Chem. Soc. 2013, 135, 9255.