Intramolecular Amido Transfer Leading to Structurally Diverse Nitrogen-Containing Macrocycles
Heejeong Kim
Department of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, 34141 Republic of Korea
Center for Catalytic Hydrocarbon Functionalization, Institute for Basic Science (IBS), Daejeon, 34141 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Dr. Sukbok Chang
Department of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, 34141 Republic of Korea
Center for Catalytic Hydrocarbon Functionalization, Institute for Basic Science (IBS), Daejeon, 34141 Republic of Korea
Search for more papers by this authorHeejeong Kim
Department of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, 34141 Republic of Korea
Center for Catalytic Hydrocarbon Functionalization, Institute for Basic Science (IBS), Daejeon, 34141 Republic of Korea
Search for more papers by this authorCorresponding Author
Prof. Dr. Sukbok Chang
Department of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, 34141 Republic of Korea
Center for Catalytic Hydrocarbon Functionalization, Institute for Basic Science (IBS), Daejeon, 34141 Republic of Korea
Search for more papers by this authorGraphical Abstract
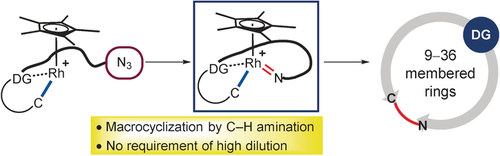
Azamacrocycles: A rhodium-catalyzed inner-sphere intramolecular C−H amination of tailormade acetophenone ketoximes tethered with either aryl or alkyl azides furnishes azamacrocyclic compounds with up to 36-membered rings. While substrates bearing aryl azides underwent a monomeric ring formation in high yields, a dimeric double cyclization took place exclusively with alkyl-azide-tethered ketoximes.
Abstract
Reported herein is the development of rhodium-catalyzed intramolecular amido transfer as an efficient route to nitrogen-containing macrocycles starting from acetophenone ketoximes tethered with either aryl or alkyl azides. Facile generation of rhodacycles and metal imido intermediates was the key to success in this mechanistic scaffold to represent the first example of an intramolecular inner-sphere C−H amination. While substrates bearing aryl azides underwent a monomeric ring formation in high yields, a dimeric double cyclization took place exclusively with alkyl-azide-tethered ketoximes, thus affording up to 36-membered azamacrocyclic products.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201700113-sup-0001-misc_information.pdf9.6 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. Hili, A. K. Yudin, Nat. Chem. Biol. 2006, 2, 284;
- 1b Amino Group Chemistry: From Synthesis to the Life Sciences, Wiley-VCH, Weinheim, 2008.
- 2For selected reviews on the C−H amination, see:
- 2aA. Armstrong, J. C. Collins, Angew. Chem. Int. Ed. 2010, 49, 2282; Angew. Chem. 2010, 122, 2332;
- 2bT. G. Driver, Org. Biomol. Chem. 2010, 8, 3831;
- 2cS. H. Cho, J. Y. Kim, J. Kwak, S. Chang, Chem. Soc. Rev. 2011, 40, 5068;
- 2dF. Collet, C. Lescot, P. Dauban, Chem. Soc. Rev. 2011, 40, 1926;
- 2eJ. L. Roizen, M. E. Harvey, J. Du Bois, Acc. Chem. Res. 2012, 45, 911;
- 2fJ. L. Jeffrey, R. Sarpong, Chem. Sci. 2013, 4, 4092;
- 2gM.-L. Louillat, F. W. Patureau, Chem. Soc. Rev. 2014, 43, 901;
- 2hK. Shin, H. Kim, S. Chang, Acc. Chem. Res. 2015, 48, 1040;
- 2iJ. Jiao, K. Murakami, K. Itami, ACS Catal. 2016, 6, 610.
- 3For selected reviews on the C−H functionalization, see:
- 3aI. V. Seregin, V. Gevorgyan, Chem. Soc. Rev. 2007, 36, 1173;
- 3bH. M. L. Davies, J. R. Manning, Nature 2008, 451, 417;
- 3cM. M. Díaz-Requejo, P. J. Pérez, Chem. Rev. 2008, 108, 3379;
- 3dD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 3eT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 3fH. Lu, X. P. Zhang, Chem. Soc. Rev. 2011, 40, 1899;
- 3gJ. Wencel-Delord, T. Droge, F. Liu, F. Glorius, Chem. Soc. Rev. 2011, 40, 4740;
- 3hL. McMurray, F. O'Hara, M. J. Gaunt, Chem. Soc. Rev. 2011, 40, 1885;
- 3iT.-S. Mei, L. Kou, S. Ma, K. M. Engle, J.-Q. Yu, Synthesis 2012, 44, 1778.
- 4
- 4aR. H. Crabtree, J. Chem. Soc. Dalton Trans. 2001, 2437;
- 4bA. R. Dick, M. S. Sanford, Tetrahedron 2006, 62, 2439.
- 5
- 5aZ. Li, D. A. Capretto, R. Rahaman, C. He, Angew. Chem. Int. Ed. 2007, 46, 5184; Angew. Chem. 2007, 119, 5276;
- 5bK. W. Fiori, J. Du Bois, J. Am. Chem. Soc. 2007, 129, 562;
- 5cE. T. Hennessy, T. A. Betley, Science 2013, 340, 591.
- 6
- 6aL. He, P. W. H. Chan, W.-M. Tsui, W.-Y. Yu, C.-M. Che, Org. Lett. 2004, 6, 2405;
- 6bZ. Li, D. A. Capretto, R. O. Rahaman, C. He, J. Am. Chem. Soc. 2007, 129, 12058;
- 6cM. P. Paudyal, A. M. Adebesin, S. R. Burt, D. H. Ess, Z. Ma, L. Kürti, J. R. Falck, Science 2016, 353, 1144.
- 7
- 7aB. J. Stokes, H. J. Dong, B. E. Leslie, A. L. Pumphrey, T. G. Driver, J. Am. Chem. Soc. 2007, 129, 7500;
- 7bJ. Bonnamour, C. Bolm, Org. Lett. 2011, 13, 2012;
- 7cI. T. Alt, B. Plietker, Angew. Chem. Int. Ed. 2016, 55, 1519; Angew. Chem. 2016, 128, 1542;
- 7dM. E. Harvey, D. G. Musaev, J. Du Bois, J. Am. Chem. Soc. 2011, 133, 17207.
- 8
- 8aH.-Y. Thu, W.-Y. Yu, C.-M. Che, J. Am. Chem. Soc. 2006, 128, 9048;
- 8bX. Huang, Y. Wang, J. Lan, J. You, Angew. Chem. Int. Ed. 2015, 54, 9404; Angew. Chem. 2015, 127, 9536;
- 8cB. Liu, B. Li, B. Wang, Chem. Commun. 2015, 51, 16334;
- 8dH. Kim, G. Park, J. Park, S. Chang, ACS Catal. 2016, 6, 5922;
- 8eX.-H. Hu, X.-F. Yang, T.-P. Loh, ACS Catal. 2016, 6, 5930.
- 9
- 9aW. C. P. Tsang, N. Zheng, S. L. Buchwald, J. Am. Chem. Soc. 2005, 127, 14560;
- 9bT.-S. Mei, X. Wang, J.-Q. Yu, J. Am. Chem. Soc. 2009, 131, 10806;
- 9cS. H. Cho, J. Yoon, S. Chang, J. Am. Chem. Soc. 2011, 133, 5996;
- 9dK. Takamatsu, K. Hirano, T. Satoh, M. Miura, Org. Lett. 2014, 16, 2892;
- 9eZ. Wang, J. Ni, Y. Kuninobu, M. Kanai, Angew. Chem. Int. Ed. 2014, 53, 3496; Angew. Chem. 2014, 126, 3564;
- 9fG. He, G. Lu, Z. Guo, P. Liu, G. Chen, Nat. Chem. 2016, 8, 1131.
- 10For selected reviews on the conventional macrocyclization reactions, see:
- 10aE. Arundale, L. A. Mikeska, Chem. Rev. 1952, 51, 505;
- 10bG. S. C. Srikanth, S. L. Castle, Tetrahedron 2005, 61, 10377;
- 10cK. C. Nicolaou, P. G. Bulger, D. Sarlah, Angew. Chem. Int. Ed. 2005, 44, 4490; Angew. Chem. 2005, 117, 4564;
- 10dI. Shiina, Chem. Rev. 2007, 107, 239.
- 11
- 11aK. J. Fraunhoffer, N. Prabagaran, L. E. Sirois, M. C. White, J. Am. Chem. Soc. 2006, 128, 9032;
- 11bE. M. Stang, M. C. White, Nat. Chem. 2009, 1, 547;
- 11cE. M. Stang, M. C. White, Angew. Chem. Int. Ed. 2011, 50, 2094; Angew. Chem. 2011, 123, 2142.
- 12
- 12aA. Lumbroso, N. Abermil, B. Breit, Chem. Sci. 2012, 3, 789;
- 12bH. Dong, C. Limberakis, S. Liras, D. Price, K. James, Chem. Commun. 2012, 48, 11644;
- 12cL. Mendive-Tapia, S. Preciado, J. Garcia, R. Ramon, N. Kielland, F. Albericio, R. Lavilla, Nat. Commun. 2015, 6, 7160.
- 13
- 13aJ. Ryu, K. Shin, S. H. Park, J. Y. Kim, S. Chang, Angew. Chem. Int. Ed. 2012, 51, 9904; Angew. Chem. 2012, 124, 10042;
- 13bK. Shin, Y. Baek, S. Chang, Angew. Chem. Int. Ed. 2013, 52, 8031; Angew. Chem. 2013, 125, 8189;
- 13cS. H. Park, J. Kwak, K. Shin, J. Ryu, Y. Park, S. Chang, J. Am. Chem. Soc. 2014, 136, 2492.
- 14
- 14aL. V. Desai, K. L. Hull, M. S. Sanford, J. Am. Chem. Soc. 2004, 126, 9542;
- 14bZ. Ren, F. Mo, G. Dong, J. Am. Chem. Soc. 2012, 134, 16991;
- 14cT. Kang, H. Kim, J. G. Kim, S. Chang, Chem. Commun. 2014, 50, 12073.
- 15
- 15aM. E. Maier, Angew. Chem. Int. Ed. 2000, 39, 2073;
10.1002/1521-3773(20000616)39:12<2073::AID-ANIE2073>3.0.CO;2-0 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2000, 112, 2153;
- 15bM. Nevalainen, A. M. P. Koskinen, Angew. Chem. Int. Ed. 2001, 40, 4060;
10.1002/1521-3773(20011105)40:21<4060::AID-ANIE4060>3.0.CO;2-8 CAS PubMed Web of Science® Google ScholarAngew. Chem. 2001, 113, 4184;
- 15c Modern Physical Organic Chemistry, University Science Books, Sausalito, CA, 2006.
- 16For recent literature on metal-mediated imine formation from alkyl azides, see:
- 16aG. Albertin, S. Antoniutti, D. Baldan, J. Castro, S. García-Fontán, Inorg. Chem. 2008, 47, 742;
- 16bJ. H. Lee, S. Gupta, W. Jeong, Y. H. Rhee, J. Park, Angew. Chem. Int. Ed. 2012, 51, 10851; Angew. Chem. 2012, 124, 11009.
- 17Applications of azamacrocycles to coordination chemistry and molecular recognitions:
- 17aP. V. Bernhardt, G. A. Lawrance, Coord. Chem. Rev. 1990, 104, 297;
- 17bD.-C. Zhong, T.-B. Lu, Chem. Commun. 2016, 52, 10322.
- 18CCDC 1525344 (4 a), 1525345 (4 h), 1525349 (4 i), 1525347 (7), 1525348 (9 b), and 1525346 (9 g) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.