Enantioselective Aza-Ene-type Reactions of Enamides with Gold Carbenes Generated from α-Diazoesters
Feng Zhao
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorNan Li
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorTao Zhang
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorZhi-Yong Han
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Shi-Wei Luo
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Liu-Zhu Gong
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300072 China
Search for more papers by this authorFeng Zhao
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorNan Li
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
These authors contributed equally to this work.
Search for more papers by this authorTao Zhang
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorZhi-Yong Han
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Shi-Wei Luo
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Search for more papers by this authorCorresponding Author
Prof. Dr. Liu-Zhu Gong
Hefei National Laboratory for Physical Sciences at the Microscale and Department of Chemistry, University of Science and Technology of China, Hefei, 230026 China
Collaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300072 China
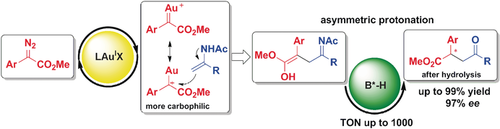
Search for more papers by this authorGraphical Abstract
Abstract
Carbophilic gold carbenes generated from the decomposition of α-diazoesters show high reactivity towards enamides, leading to an unprecedented aza-ene-type reaction. The presence of 0.1 mol % of a chiral Brønsted acid co-catalyst is sufficient to give synthetically relevant γ-keto esters in excellent yields and selectivities (up to 99 % yield, 97 % ee).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201612208-sup-0001-misc_information.pdf4.5 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aR. R. Torres, Stereoselective Organocatalysis: Bond Formation Methodologies and Activation Modes, Wiley, Hoboken, 2013;
10.1002/9781118604755 Google Scholar
- 1bE. M. Carreira, L. Kvaerno, Classics in Stereoselective Synthesis, Wiley-VCH, Weinheim, 2009.
- 2
- 2aT. Curtius, Ber. Dtsch. Chem. Ges. 1883, 16, 2230;
10.1002/cber.188301602136 Google Scholar
- 2bG. Maas, Angew. Chem. Int. Ed. 2009, 48, 8186; Angew. Chem. 2009, 121, 8332.
- 3For selected reviews, see:
- 3aM. Regitz, G. Maas, Diazo Compounds-Properties and Synthesis, Academic Press, Orlando, 1986;
- 3bM. P. Doyle, M. A. McKervey, T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley, New York, 1998;
- 3cA. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire, M. A. McKervey, Chem. Rev. 2015, 115, 9981;
- 3dN. R. Candeias, R. Paterna, P. M. P. Gois, Chem. Rev. 2016, 116, 2937.
- 4
- 4aA. Padwa, M. D. Weingarten, Chem. Rev. 1996, 96, 223;
- 4bM. P. Doyle, D. C. Forbes, Chem. Rev. 1998, 98, 911;
- 4cD. M. Hodgson, F. Y. T. M. Pierard, P. A. Stupple, Chem. Soc. Rev. 2001, 30, 50;
- 4dH. M. L. Davies, R. E. J. Beckwith, Chem. Rev. 2003, 103, 2861;
- 4eD. Gillingham, N. Fei, Chem. Soc. Rev. 2013, 42, 4918.
- 5For early reports, see:
- 5aW. R. Moser, J. Am. Chem. Soc. 1969, 91, 1135;
- 5bH. Nozaki, S. Moriuti, M. Yamabe, R. Noyori, Tetrahedron Lett. 1966, 7, 59;
10.1016/S0040-4039(01)99630-3 Google Scholar
- 5cN. Petiniot, A. J. Anciaux, A. F. Noels, A. J. Hubert, P. Teyssié, Tetrahedron Lett. 1978, 19, 1239;
10.1016/S0040-4039(01)94511-3 Google Scholar
- 5dM. P. Doyle, M. Protopopova, P. Müller, D. Ene, E. A. Shapiro, J. Am. Chem. Soc. 1994, 116, 8492.
- 6S.-F. Zhu, Q.-L. Zhou, Acc. Chem. Res. 2012, 45, 1365.
- 7For a review, see: G. Mehta, S. Muthusamy, Tetrahedron 2002, 58, 9477.
- 8X. Guo, W. Hu, Acc. Chem. Res. 2013, 46, 2427.
- 9
- 9aQ. Xiao, Y. Zhang, J. Wang, Acc. Chem. Res. 2013, 46, 236;
- 9bY. Zhang, J. Wang, Eur. J. Org. Chem. 2011, 1015;
- 9cH. Li, Y. Zhang, J. Wang, Synthesis 2013, 45, 3090.
- 10
- 10aM. R. Fructos, T. R. Belderrain, P. de Frémont, N. M. Scott, S. P. Nolan, M. M. Díaz-Requejo, P. J. Pérez, Angew. Chem. Int. Ed. 2005, 44, 5284; Angew. Chem. 2005, 117, 5418;
- 10bD. Benitez, N. D. Shapiro, E. Tkatchouk, Y. Wang, W. A. Goddard III, F. D. Toste, Nat. Chem. 2009, 1, 482;
- 10cY. Xi, Y. Su, Z. Yu, B. Dong, E. J. McClain, Y. Lan, X. Shi, Angew. Chem. Int. Ed. 2014, 53, 9817; Angew. Chem. 2014, 126, 9975;
- 10dZ. Yu, B. Ma, M. Chen, H.-H. Wu, L. Liu, J. Zhang, J. Am. Chem. Soc. 2014, 136, 6904.
- 11Y. Wang, M. E. Muratore, A. M. Echavarren, Chem. Eur. J. 2015, 21, 7332.
- 12
- 12aS. Shin, Top. Curr. Chem. 2014, 357, 25;
- 12bL. Zhang, Acc. Chem. Res. 2014, 47, 877;
- 12cH.-S. Yeom, S. Shin, Acc. Chem. Res. 2014, 47, 966.
- 13For asymmetric addition reactions, see:
- 13aM. Terada, K. Machioka, K. Sorimachi, Angew. Chem. Int. Ed. 2006, 45, 2254; Angew. Chem. 2006, 118, 2312;
- 13bM. Terada, K. Machioka, K. Sorimachi, J. Am. Chem. Soc. 2007, 129, 10336;
- 13cL. Zu, H. Xie, H. Li, J. Wang, X. Yu, W. Wang, Chem. Eur. J. 2008, 14, 6333;
- 13dM. Terada, K. Soga, N. Momiyama, Angew. Chem. Int. Ed. 2008, 47, 4122; Angew. Chem. 2008, 120, 4190;
- 13eK. Zheng, X. Liu, J. Zhao, Y. Yang, L. Lin, X. Feng, Chem. Commun. 2010, 46, 3771;
- 13fA. Noole, M. Borissova, M. Lopp, T. Kanger, J. Org. Chem. 2011, 76, 1538; for asymmetric substitution reactions, see:
- 13gW. Tan, B.-X. Du, X. Li, X. Zhu, F. Shi, S.-J. Tu, J. Org. Chem. 2014, 79, 4635;
- 13hC. Guo, J. Song, J.-Z. Huang, P.-H. Chen, S.-W. Luo, L.-Z. Gong, Angew. Chem. Int. Ed. 2012, 51, 1046; Angew. Chem. 2012, 124, 1070;
- 13iQ.-X. Guo, Y.-G. Peng, J.-W. Zhang, L. Song, Z. Feng, L.-Z. Gong, Org. Lett. 2009, 11, 4620.
- 14For leading reports, see:
- 14aZ.-Y. Han, H. Xiao, X.-H. Chen, L.-Z. Gong, J. Am. Chem. Soc. 2009, 131, 9182;
- 14bM. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt, D. J. Dixon, J. Am. Chem. Soc. 2009, 131, 10796;
- 14cX.-Y. Liu, C.-M. Che, Org. Lett. 2009, 11, 4204; for reviews, see:
- 14dC. C. J. Loh, D. Enders, Chem. Eur. J. 2012, 18, 10212;
- 14eX. Wu, M.-L. Li, L.-Z. Gong, Acta Chim. Sin. 2013, 71, 1091;
- 14fN. T. Patil, V. S. Raut, R. B. Tella, Chem. Commun. 2013, 49, 570;
- 14gD.-F. Chen, Z.-Y. Han, X.-L. Zhou, L.-Z. Gong, Acc. Chem. Res. 2014, 47, 2365.
- 15
- 15aC. H. Cheon, H. Yamamoto, J. Am. Chem. Soc. 2008, 130, 9246;
- 15bB. Xu, S.-F. Zhu, X.-L. Xie, J.-J. Shen, Q.-L. Zhou, Angew. Chem. Int. Ed. 2011, 50, 11483; Angew. Chem. 2011, 123, 11685;
- 15cB. Xu, S.-F. Zhu, Z.-C. Zhang, Z.-X. Yu, Y. Ma, Q.-L. Zhou, Chem. Sci. 2014, 5, 1442;
- 15dB. Xu, M.-L. Li, X.-D. Zuo, S.-F. Zhu, Q.-L. Zhou, J. Am. Chem. Soc. 2015, 137, 8700.
- 16For catalytic asymmetric syntheses of γ-keto esters, see:
- 16aG. K. S. Prakash, F. Wang, Z. Zhang, C. Ni, R. Haiges, G. A. Olah, Org. Lett. 2012, 14, 3260;
- 16bK. S. Yang, A. E. Nibbs, Y. E. Türkmen, V. H. Rawal, J. Am. Chem. Soc. 2013, 135, 16050.
- 17
- 17aT. Akiyama, J. Itoh, K. Yokota, K. Fuchibe, Angew. Chem. Int. Ed. 2004, 43, 1566; Angew. Chem. 2004, 116, 1592;
- 17bD. Uraguchi, M. Terada, J. Am. Chem. Soc. 2004, 126, 5356;
- 17cM. Terada, Synthesis 2010, 1929;
- 17dT. Akiyama, Chem. Rev. 2007, 107, 5744;
- 17eD. Kampen, C. M. Reisinger, B. List, Top. Curr. Chem. 2009, 291, 395;
- 17fJ. Yu, F. Shi, L.-Z. Gong, Acc. Chem. Res. 2011, 44, 1156;
- 17gD. Parmar, E. Sugiono, S. Raja, M. Rueping, Chem. Rev. 2014, 114, 9047;
- 17hT. Akiyama, K. Mori, Chem. Rev. 2015, 115, 9277.
- 18M. J. Frisch et al. Gaussian 09, Revision A.02, Gaussian, Inc.: Wallingford, CT., 2009 (see the Supporting Information for details).
- 19For DFT calculations on chiral phosphoric acid catalysis, see: J. P. Reid, L. Simón, J. M. Goodman, Acc. Chem. Res. 2016, 49, 1029.
- 20Y. Liu, Z. Yu, J. Z. Zhang, L. Liu, F. Xia, J. Zhang, Chem. Sci. 2016, 7, 1988.
- 21A. D. Rodríguez, O. M. Cóbar, O. L. Padilla, J. Nat. Prod. 1997, 60, 915.