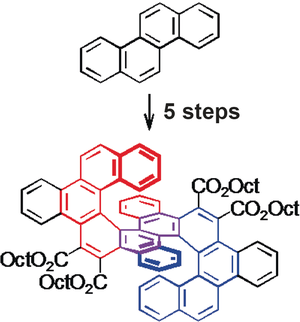
A Naphtho-Fused Double [7]Helicene from a Maleate-Bridged Chrysene Trimer
Dr. Marli Ferreira
Centre de Recherche Paul Pascal, CNRS, 115, av. Schweitzer, 33600 Pessac, France
Departamento de Química, Universidade Federal de Santa Catarina, CEP, 88040-900 Florianópolis, Santa Catarina, Brazil
Search for more papers by this authorGuillaume Naulet
Centre de Recherche Paul Pascal, Université de Bordeaux, 115, av. Schweitzer, 33600 Pessac, France
Search for more papers by this authorProf. Hugo Gallardo
Departamento de Química, Universidade Federal de Santa Catarina, CEP, 88040-900 Florianópolis, Santa Catarina, Brazil
Search for more papers by this authorDr. Pierre Dechambenoit
Centre de Recherche Paul Pascal, Université de Bordeaux, 115, av. Schweitzer, 33600 Pessac, France
Search for more papers by this authorCorresponding Author
Dr. Harald Bock
Centre de Recherche Paul Pascal, CNRS, 115, av. Schweitzer, 33600 Pessac, France
Search for more papers by this authorCorresponding Author
Dr. Fabien Durola
Centre de Recherche Paul Pascal, CNRS, 115, av. Schweitzer, 33600 Pessac, France
Search for more papers by this authorDr. Marli Ferreira
Centre de Recherche Paul Pascal, CNRS, 115, av. Schweitzer, 33600 Pessac, France
Departamento de Química, Universidade Federal de Santa Catarina, CEP, 88040-900 Florianópolis, Santa Catarina, Brazil
Search for more papers by this authorGuillaume Naulet
Centre de Recherche Paul Pascal, Université de Bordeaux, 115, av. Schweitzer, 33600 Pessac, France
Search for more papers by this authorProf. Hugo Gallardo
Departamento de Química, Universidade Federal de Santa Catarina, CEP, 88040-900 Florianópolis, Santa Catarina, Brazil
Search for more papers by this authorDr. Pierre Dechambenoit
Centre de Recherche Paul Pascal, Université de Bordeaux, 115, av. Schweitzer, 33600 Pessac, France
Search for more papers by this authorCorresponding Author
Dr. Harald Bock
Centre de Recherche Paul Pascal, CNRS, 115, av. Schweitzer, 33600 Pessac, France
Search for more papers by this authorCorresponding Author
Dr. Fabien Durola
Centre de Recherche Paul Pascal, CNRS, 115, av. Schweitzer, 33600 Pessac, France
Search for more papers by this authorGraphical Abstract
Abstract
Perkin condensation of chrysenyl-6-acetic acid with chrysenylene-6,12-diglyoxylic acid followed by in situ esterification gives a bismaleate, whose conjugated stilbene moieties are efficiently shielded against intermolecular condensations and undergo iodine-catalyzed oxidative photocyclization in toluene without the need for high dilution. The concentration is limited by the low solubility of the flexible bismaleate at room temperature. The so-obtained double [7]helicene crystallizes in a nonchiral meso form. It is notably more soluble than its flexible precursor because it cannot fold to optimize π–π stacking.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201610793-sup-0001-misc_information.pdf862.5 KB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Celis, M. N. Nair, A. Taleb-Ibrahimi, E. H. Conrad, C. Berger, W. A. de Heer, A. Tejeda, J. Phys. D 2016, 49, 143001;
- 1bL. Chen, Y. Hernandez, X. Feng, K. Müllen, Angew. Chem. Int. Ed. 2012, 51, 7640; Angew. Chem. 2012, 124, 7758;
- 1cL. Ma, J. Wang, F. Ding, ChemPhysChem 2013, 14, 47.
- 2
- 2aY.-W. Son, M. L. Cohen, S. G. Louie, Phys. Rev. Lett. 2006, 97, 216803;
- 2bF. B. Mallory, K. E. Butler, A. C. Evans, C. W. Mallory, Tetrahedron Lett. 1996, 37, 7173;
- 2cF. B. Mallory, K. E. Butler, A. C. Evans, E. J. Brondyke, C. W. Mallory, C. Yang, A. Ellenstein, J. Am. Chem. Soc. 1997, 119, 2119.
- 3K. Mori, T. Murase, M. Fujita, Angew. Chem. Int. Ed. 2015, 54, 6847; Angew. Chem. 2015, 127, 6951.
- 4
- 4aR. H. Martin, M. Baes, Tetrahedron 1975, 31, 2135;
- 4bY. Shen, C.-F. Chen, Chem. Rev. 2012, 112, 1463;
- 4cM. Gingras, Chem. Soc. Rev. 2013, 42, 968;
- 4dM. Gingras, G. Félix, R. Peresutti, Chem. Soc. Rev. 2013, 42, 1007;
- 4eM. Gingras, Chem. Soc. Rev. 2013, 42, 1051.
- 5
- 5aW. H. Laarhoven, T. H. J. M. Cuppen, Recueil Trav. Chim. Pays-Bas 1973, 92, 553;
- 5bW. H. Laarhoven, M. H. De Jong, Recueil Trav. Chim. Pays-Bas 1973, 92, 651;
- 5cR. H. Martin, C. Eyndels, N. Defay, Tetrahedron 1974, 30, 3339.
- 6D. Peña, A. Cobas, D. Pérez, E. Guitián, L. Castedo, Org. Lett. 2003, 5, 1863.
- 7
- 7aT. Fujikawa, Y. Segawa, K. Itami, J. Am. Chem. Soc. 2015, 137, 7763;
- 7bY. Hu, X.-Y. Wang, P.-X. Peng, X.-C. Wang, X.-Y. Cao, X. Feng, K. Müllen, A. Narita, Angew. Chem. Int. Ed. DOI: 10.1002/anie.201610434; Angew. Chem. DOI: 10.1002/ange.201610434.
- 8
- 8aC. F. Koelsch, S. Wawzonek, J. Org. Chem. 1941, 6, 684;
- 8bE. K. Fields, S. J. Behrend, S. Meyerson, M. L. Winzenburg, B. R. Ortega, H. K. Hall, Jr., J. Org. Chem. 1990, 55, 5165.
- 9H. Bock, D. Subervie, P. Mathey, A. Pradhan, P. Sarkar, P. Dechambenoit, E. A. Hillard, F. Durola, Org. Lett. 2014, 16, 1546 and H. Bock, D. Subervie, P. Mathey, A. Pradhan, P. Sarkar, P. Dechambenoit, E. A. Hillard, F. Durola, Org. Lett. 2014, 16, 2573.
- 10H. Bock, S. Huet, P. Dechambenoit, E. A. Hillard, F. Durola, Eur. J. Org. Chem. 2015, 1033.
- 11J. E. Milne, T. Storz, J. T. Coyler, O. R. Thiel, M. D. Seran, R. D. Larsen, J. A. J. Murry, J. Org. Chem. 2011, 76, 9519.
- 12
- 12aS. K. Collins, A. Grandbois, M. P. Vachon, J. Côté, Angew. Chem. Int. Ed. 2006, 45, 2923; Angew. Chem. 2006, 118, 2989; also compare:
- 12bR. H. Martin, N. Defay, H. P. Figeys, M. Flammang-Barbieux, J. P. Cosyn, M. Gelbcke, J. J. Schurter, Tetrahedron 1969, 25, 4985;
- 12cR. H. Martin, N. Defay, H. P. Figeys, K. Lê van, J. J. Ruelle, J. J. Schurter, Helv. Chim. Acta 1972, 55, 2241.
- 13
- 13aM. J. Fuchter, M. Weimar, X. Yang, D. K. Judge, A. J. P. White, Tetrahedron Lett. 2012, 53, 1108;
- 13bM. Joly, N. Defay, R. H. Martin, J. P. Declercq, G. Germain, B. Soubrier-Payen, M. van Meerssche, Helv. Chim. Acta 1977, 60, 537;
- 13cT. E. M. van den Hark, P. T. Beurskens, Cryst. Struct. Commun. 1976, 5, 247;
- 13dP. T. Beurskens, G. Beurskens, T. E. M. van den Hark, Cryst. Struct. Commun. 1976, 5, 241.