Internally 2,5-Thienylene-Bridged [46]Decaphyrin: (Annuleno)annulene Network Consisting of Möbius Aromatic Thia[28]hexaphyrins and Strong Hückel Aromaticity of its Protonated Form
Takanori Soya
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorDr. Hirotaka Mori
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorYongseok Hong
Spectroscopy Laboratory for Functional π-Electronic Systems and Department of Chemistry, Yonsei University, Seoul, 120-749 Korea
Search for more papers by this authorYun Hee Koo
Spectroscopy Laboratory for Functional π-Electronic Systems and Department of Chemistry, Yonsei University, Seoul, 120-749 Korea
Search for more papers by this authorCorresponding Author
Prof. Dr. Dongho Kim
Spectroscopy Laboratory for Functional π-Electronic Systems and Department of Chemistry, Yonsei University, Seoul, 120-749 Korea
Search for more papers by this authorCorresponding Author
Prof. Dr. Atsuhiro Osuka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorTakanori Soya
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorDr. Hirotaka Mori
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorYongseok Hong
Spectroscopy Laboratory for Functional π-Electronic Systems and Department of Chemistry, Yonsei University, Seoul, 120-749 Korea
Search for more papers by this authorYun Hee Koo
Spectroscopy Laboratory for Functional π-Electronic Systems and Department of Chemistry, Yonsei University, Seoul, 120-749 Korea
Search for more papers by this authorCorresponding Author
Prof. Dr. Dongho Kim
Spectroscopy Laboratory for Functional π-Electronic Systems and Department of Chemistry, Yonsei University, Seoul, 120-749 Korea
Search for more papers by this authorCorresponding Author
Prof. Dr. Atsuhiro Osuka
Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto, 606-8502 Japan
Search for more papers by this authorGraphical Abstract
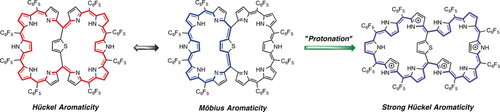
Switching aromaticity: 1,3-Phenylene- and 2,5-thienylene-bridged [46]decaphyrins A and B have been synthesized. While A shows Hückel-aromatic character derived from its global 46π-conjugated circuit, B displays an (annuleno)annulene-type structure consisting of two twisted Möbius-aromatic thia[28]hexaphyrins. Upon protonation, these [46]decaphyrins undergo large structural changes and become strongly aromatic.
Abstract
Internally 1,3-phenylene- and 2,5-thienylene-bridged [46]decaphyrins 2 and 3 have been synthesized. While 2 shows modest aromatic character derived from the global 46π-conjugated circuit, 3 displays larger aromatic character owing to the contribution of an (annuleno)annulene-type network consisting of two twisted Möbius aromatic thia[28]hexaphyrin segments in addition to the global 46π-network. Upon protonation, these [46]decaphyrins underwent large structural changes to acquire strong aromaticity. Protonated 3 has been revealed to take on a planar structure composed of fused two triangular thia[28]hexaphyrin segments.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201700607-sup-0001-misc_information.pdf39.9 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. L. Sessler, D. Seidel, Angew. Chem. Int. Ed. 2003, 42, 5134; Angew. Chem. 2003, 115, 5292;
- 1bM. Stępień, N. Sprutta, L. Latos-Grażyński, Angew. Chem. Int. Ed. 2011, 50, 4288; Angew. Chem. 2011, 123, 4376;
- 1cS. Saito, A. Osuka, Angew. Chem. Int. Ed. 2011, 50, 4342; Angew. Chem. 2011, 123, 4432;
- 1dT. Tanaka, A. Osuka, Chem. Rev. 2017, DOI: 10.1021/acs.chemrev.6b00371.
- 2
- 2aV. G. Anand, S. Saito, S. Shimizu, A. Osuka, Angew. Chem. Int. Ed. 2005, 44, 7244; Angew. Chem. 2005, 117, 7410;
- 2bG. Karthik, M. Sneha, V. P. Raja, J. M. Lim, D. Kim, A. Srinivasan, T. K. Chandrashekar, Chem. Eur. J. 2013, 19, 1886;
- 2cH. Mori, J. M. Lim, D. Kim, A. Osuka, Angew. Chem. Int. Ed. 2013, 52, 12997; Angew. Chem. 2013, 125, 13235.
- 3
- 3aM. Nakagawa, The Chemistry of Annulenes-From the Standpoint of Organic Chemistry, Osaka University Press, Osaka, 1996;
- 3bM. Nakagawa, Angew. Chem. Int. Ed. Engl. 1979, 18, 202; Angew. Chem. 1979, 91, 215.
- 4S.-i. Tamaru, L. Yu, W. J. Youngblood, K. Muthukumaran, M. Taniguchi, J. S. Lindsey, J. Org. Chem. 2004, 69, 765.
- 5
- 5aCrystallographic data for 3: C102H28F40N10S, Mr=2185.40; triclinic; space group
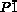 (No.2), a=18.0942(7), b=18.1757(14), c=21.3086(19) Å; α=89.292(12), β=72.729(1), γ=66.454(15)°; V=6196.8(8) Å3; ρcalcd=1.171 g cm−3; Z=2; R1=0.0840 [I>2.0σ(I)], wR2=0.2875 (all data), GOF=1.035.
(No.2), a=18.0942(7), b=18.1757(14), c=21.3086(19) Å; α=89.292(12), β=72.729(1), γ=66.454(15)°; V=6196.8(8) Å3; ρcalcd=1.171 g cm−3; Z=2; R1=0.0840 [I>2.0σ(I)], wR2=0.2875 (all data), GOF=1.035.
- 5bCrystallographic data for 4: C102H26F40N10S⋅2.5(C6)⋅(CCl2), Mr=2446.45; triclinic; space group
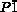 (No.2), a=15.7785(12), b=19.6157(7), c=22.386(2) Å; α=66.210(13), β=77.68(2), γ=66.653(19)°; V=5809.0(12) Å3; ρcalcd=1.399 g cm−3; Z=2; R1=0.0965 [I>2.0σ(I)], wR2=0.2888 (all data), GOF=1.024.
(No.2), a=15.7785(12), b=19.6157(7), c=22.386(2) Å; α=66.210(13), β=77.68(2), γ=66.653(19)°; V=5809.0(12) Å3; ρcalcd=1.399 g cm−3; Z=2; R1=0.0965 [I>2.0σ(I)], wR2=0.2888 (all data), GOF=1.024.
- 5cCrystallographic data for diprotonated 3: C102H30F40N10S⋅2.35(C2F3O2)⋅7.81(CO)⋅0.57(CCl2), Mr=2476.54; triclinic; space group
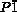 (No.2), a=13.3846(17), b=19.1244(7), c=25.590(3) Å; α=76.615(16), β=89.57(2), γ=70.103(11)°; V=5845.5(12) Å3; ρcalcd=1.549 g cm−3; Z=2; R1=0.0872 [I>2.0σ(I)], wR2=0.2753 (all data), GOF=1.033. CCDC 1524346 (3), 1524347 (4), 1524348 (diprotonated 3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre. The contributions to the scattering arising from the presence of the disordered solvents in the crystal of 3 were removed by use of the utility SQUEEZE in the PLATON software package. See;
(No.2), a=13.3846(17), b=19.1244(7), c=25.590(3) Å; α=76.615(16), β=89.57(2), γ=70.103(11)°; V=5845.5(12) Å3; ρcalcd=1.549 g cm−3; Z=2; R1=0.0872 [I>2.0σ(I)], wR2=0.2753 (all data), GOF=1.033. CCDC 1524346 (3), 1524347 (4), 1524348 (diprotonated 3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre. The contributions to the scattering arising from the presence of the disordered solvents in the crystal of 3 were removed by use of the utility SQUEEZE in the PLATON software package. See;
- 5dA. L. Spek, PLATON, A Multipurpose Crystallographic Tool, Utrecht University, Utrecht, The Netherlands, 2005;
- 5eP. van der Sluis, A. L. Spek, Acta Crystallogr. Sect. A 1990, 46, 194.
- 6Protonation of expanded porphyrins:
- 6aS. Shimizu, R. Taniguchi, A. Osuka, Angew. Chem. Int. Ed. 2005, 44, 2225; Angew. Chem. 2005, 117, 2265;
- 6bS. Saito, J.-Y. Shin, J. M. Lim, K. S. Kim, D. Kim, A. Osuka, Angew. Chem. Int. Ed. 2008, 47, 9657; Angew. Chem. 2008, 120, 9803;
- 6cJ. M. Lim, J.-Y. Shin, Y. Tanaka, S. Saito, A. Osuka, D. Kim, J. Am. Chem. Soc. 2010, 132, 3105;
- 6dT. Koide, K. Youfu, S. Saito, A. Osuka, Chem. Commun. 2009, 6047;
- 6eS.-i. Ishida, T. Higashino, S. Mori, H. Mori, N. Aratani, T. Tanaka, J. M. Lim, D. Kim, A. Osuka, Angew. Chem. Int. Ed. 2014, 53, 3427; Angew. Chem. 2014, 126, 3495;
- 6fZ. Zhang, W.-Y. Cha, N. J. Williams, E. L. Rush, M. Ishida, V. M. Lynch, D. Kim, J. L. Sessler, J. Am. Chem. Soc. 2014, 136, 7591;
- 6gT. Soya, W. Kim, D. Kim, A. Osuka, Chem. Eur. J. 2015, 21, 8341;
- 6hK. Naoda, H. Mori, J. Oh, K. H. Park, D. Kim, A. Osuka, J. Org. Chem. 2015, 80, 11726.
- 7Preliminary crystral structure of a protonated species of 2 was also obtained, which exhibited a similar structure to that of diprotonated 3 (Figure S7-5).
- 8
- 8aP. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, N. J. R. v. E. Hommes, J. Am. Chem. Soc. 1996, 118, 6317;
- 8bZ. Chen, C. S. Wannere, C. Corminboeuf, R. T. Puchta, P. v. R. Schleyer, Chem. Rev. 2005, 105, 3842.
- 9
- 9aD. Geuenich, K. Hess, F. Köhler, R. Herges, Chem. Rev. 2005, 105, 3758;
- 9bR. Herges, D. Geuenich, J. Phys. Chem. A 2001, 105, 3214;
- 9cR. Herges, Chem. Rev. 2006, 106, 4820.
- 10
- 10aJ.-Y. Shin, M.-C. Yoon, J. M. Lim, Z. S. Yoon, A. Osuka, D. Kim, Chem. Soc. Rev. 2010, 39, 2751;
- 10bS. Cho, Z. S. Yoon, K. S. Kim, M.-C. Yoon, D.-G. Cho, J. L. Sessler, D. Kim, J. Phys. Chem. Lett. 2010, 1, 895.