On the Gold-Catalyzed Generation of Vinyl Cations from 1,5-Diynes
M. Sc. Thomas Wurm
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
These authors contributed equally.
Search for more papers by this authorM. Sc. Janina Bucher
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
These authors contributed equally.
Search for more papers by this authorSarah B. Duckworth
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorDr. Matthias Rudolph
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorDr. Frank Rominger
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Crystallographic investigation.
Search for more papers by this authorCorresponding Author
Prof. Dr. A. Stephen K. Hashmi
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, 21589 Saudi Arabia
Search for more papers by this authorM. Sc. Thomas Wurm
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
These authors contributed equally.
Search for more papers by this authorM. Sc. Janina Bucher
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
These authors contributed equally.
Search for more papers by this authorSarah B. Duckworth
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorDr. Matthias Rudolph
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Search for more papers by this authorDr. Frank Rominger
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Crystallographic investigation.
Search for more papers by this authorCorresponding Author
Prof. Dr. A. Stephen K. Hashmi
Organisch-Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany
Chemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, 21589 Saudi Arabia
Search for more papers by this authorGraphical Abstract
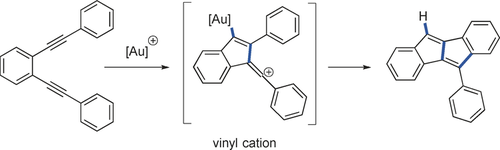
Positive intermediates: The combination of non-terminal 1,5-diynes with cationic gold complexes enables the generation of highly reactive vinyl cations that can be used for the synthesis of unsymmetrically substituted dibenzopentalenes. Quantum-chemical calculations indicate a fast valence tautomer equilibrium between a gold alkyne complex and the vinyl cation.
Abstract
Conjugated 1,5-diynes bearing two aromatic units at the alkyne termini were converted in the presence of a gold catalyst. Under mild conditions, aryl-substituted dibenzopentalenes were generated. Calculations predict that aurated vinyl cations are key intermediates of the reaction. A bidirectional approach provided selective access to the angular annulated product in high yield, which was explained by calculations.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201700057-sup-0001-misc_information.pdf9.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. M. Asiri, A. S. K. Hashmi, Chem. Soc. Rev. 2016, 45, 4471–4503;
- 1bD. Pflästerer, A. S. K. Hashmi, Chem. Soc. Rev. 2016, 45, 1331–1367;
- 1cH. Ohno, Isr. J. Chem. 2013, 53, 869–882;
- 1dN. Bongers, N. Krause, Angew. Chem. Int. Ed. 2008, 47, 2178–2181; Angew. Chem. 2008, 120, 2208–2211;
- 1eA. Fürstner, P. W. Davies, Angew. Chem. Int. Ed. 2007, 46, 3410–3449; Angew. Chem. 2007, 119, 3478–3519;
- 1fA. S. K. Hashmi, G. J. Hutchings, Angew. Chem. Int. Ed. 2006, 45, 7896–7936; Angew. Chem. 2006, 118, 8064–8105;
- 1gA. S. K. Hashmi, Gold Bull. 2004, 37, 51–65.
- 2For selected contributions, see:
- 2aN. Kim, C. Oh, A. Kim, W. Park, D. Park, Synlett 2006, 2781–2784;
- 2bJ. Zhao, C. O. Hughes, F. D. Toste, J. Am. Chem. Soc. 2006, 128, 7436–7437;
- 2cA. Das, H. K. Chang, C. H. Yang, R. S. Liu, Org. Lett. 2008, 10, 4061–4064;
- 2dC. Zhang, D.-M. Cui, L.-Y. Yao, B.-S. Wang, Y.-Z. Hu, T. Hayashi, J. Org. Chem. 2008, 73, 7811–7813;
- 2eC. Sperger, A. Fiksdahl, Org. Lett. 2009, 11, 2449–2452;
- 2fS. Kramer, J. L. H. Madsen, M. Rottländer, T. Skrydstrup, Org. Lett. 2010, 12, 2758–2761;
- 2gC. A. Sperger, A. Fiksdahl, J. Org. Chem. 2010, 75, 4542–4553;
- 2hA. S. K. Hashmi, M. Bührle, M. Wölfle, M. Rudolph, M. Wieteck, F. Rominger, W. Frey, Chem. Eur. J. 2010, 16, 9846–9854;
- 2iK. Hirano, Y. Inaba, T. Watanabe, S. Oishi, N. Fujii, H. Ohno, Adv. Synth. Catal. 2010, 352, 368–372;
- 2jA. S. K. Hashmi, T. Haeffner, M. Rudolph, F. Rominger, Chem. Eur. J. 2011, 17, 8195–8201;
- 2kK. Hirano, Y. Inaba, N. Takahashi, M. Shimano, S. Oishi, N. Fujii, H. Ohno, J. Org. Chem. 2011, 76, 1212–1227;
- 2lD.-H. Zhang, L.-F. Yao, Y. Wei, M. Shi, Angew. Chem. Int. Ed. 2011, 50, 2583–2587; Angew. Chem. 2011, 123, 2631–2635;
- 2mG. Yue, Y. Zhang, L. Fang, C.-c. Li, T. Luo, Z. Yang, Angew. Chem. Int. Ed. 2014, 53, 1837–1840; Angew. Chem. 2014, 126, 1868–1871;
- 2nW. Rao, D. Susanti, B. J. Ayers, P. W. H. Chan, J. Am. Chem. Soc. 2015, 137, 6350–6355.
- 3
- 3aA. Gómez-Suárez, S. P. Nolan, Angew. Chem. Int. Ed. 2012, 51, 8156–8159; Angew. Chem. 2012, 124, 8278–8281;
- 3bI. Braun, A. M. Asiri, A. S. K. Hashmi, ACS Catal. 2013, 3, 1902–1907;
- 3cA. S. K. Hashmi, Acc. Chem. Res. 2014, 47, 864–876;
- 3dL. Ye, Y. Wang, D. H. Aue, L. Zhang, J. Am. Chem. Soc. 2012, 134, 31–34;
- 3eA. S. K. Hashmi, I. Braun, M. Rudolph, F. Rominger, Organometallics 2012, 31, 644–661;
- 3fA. S. K. Hashmi, M. Wieteck, I. Braun, P. Nösel, L. Jongbloed, M. Rudolph, F. Rominger, Adv. Synth. Catal. 2012, 354, 555–562;
- 3gA. S. K. Hashmi, I. Braun, P. Nösel, J. Schädlich, M. Wieteck, M. Rudolph, F. Rominger, Angew. Chem. Int. Ed. 2012, 51, 4456–4460; Angew. Chem. 2012, 124, 4532–4536;
- 3hA. S. K. Hashmi, M. Wieteck, I. Braun, M. Rudolph, F. Rominger, Angew. Chem. Int. Ed. 2012, 51, 10633–10637; Angew. Chem. 2012, 124, 10785–10789;
- 3iM. M. Hansmann, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2013, 52, 2593–2598; Angew. Chem. 2013, 125, 2653–2659;
- 3jD. D. Vachhani, M. Galli, J. Jacobs, L. Van Meervelt, E. V. Van der Eycken, Chem. Commun. 2013, 49, 7171;
- 3kP. Nösel, T. Lauterbach, M. Rudolph, F. Rominger, A. S. K. Hashmi, Chem. Eur. J. 2013, 19, 8634–8641;
- 3lK. Graf, P. D. Hindenberg, Y. Tokimizu, S. Naoe, M. Rudolph, F. Rominger, H. Ohno, A. S. K. Hashmi, ChemCatChem 2014, 6, 199–204;
- 3mM. M. Hansmann, S. Tšupova, M. Rudolph, F. Rominger, A. S. K. Hashmi, Chem. Eur. J. 2014, 20, 2215–2223;
- 3nM. H. Vilhelmsen, A. S. K. Hashmi, Chem. Eur. J. 2014, 20, 1901–1908;
- 3oM. Wieteck, Y. Tokimizu, M. Rudolph, F. Rominger, H. Ohno, N. Fujii, A. S. K. Hashmi, Chem. Eur. J. 2014, 20, 16331–16336;
- 3pM. H. Larsen, K. N. Houk, A. S. K. Hashmi, J. Am. Chem. Soc. 2015, 137, 10668–10676;
- 3qC. Yu, B. Chen, T. Zhou, Q. Tian, G. Zhang, Angew. Chem. Int. Ed. 2015, 54, 10903–10907; Angew. Chem. 2015, 127, 11053–11057.
- 4J. Bucher, T. Wurm, K. S. Nalivela, M. Rudolph, F. Rominger, A. S. K. Hashmi, Angew. Chem. Int. Ed. 2014, 53, 3854–3858; Angew. Chem. 2014, 126, 3934–3939.
- 5For the generation of vinyl cations by gold catalysis, see also:
- 5aR. Balamurugan, V. Gudla, Org. Lett. 2009, 11, 3116–3119;
- 5bY. Odabachian, X. F. Le Goff, F. Gagosz, Chem. Eur. J. 2009, 15, 8966–8970;
- 5cJ. Schädlich, T. Lauterbach, M. Rudolph, F. Rominger, A. S. K. Hashmi, J. Organomet. Chem. 2015, 795, 71–77;
- 5dE. Rettenmeier, M. M. Hansmann, A. Ahrens, K. Rübenacker, T. Saboo, J. Massholder, C. Meier, M. Rudolph, F. Rominger, A. S. K. Hashmi, Chem. Eur. J. 2015, 21, 14401–14409;
- 5eK. Ji, X. Liu, B. Du, F. Yang, J. Gao, Chem. Commun. 2015, 51, 10318–10321.
- 6
- 6aE. Müller, K. Munk, P. Ziemek, M. Sauerbier, Liebigs Ann. Chem. 1968, 713, 40–48;
- 6bE. Müller, K. Munk, H.-G. Fritz, M. Sauerbier, Liebigs Ann. Chem. 1969, 723, 76–82;
- 6cE. Müller, J. Heiß, M. Sauerbier, Liebigs Ann. Chem. 1969, 723, 61–75;
- 6dY. Badrieh, J. Blum, I. Amer, K. P. C. Vollhardt, J. Mol. Catal. 1991, 66, 295–312;
- 6eJ.-J. Lian, P.-C. Chen, Y.-P. Lin, H.-C. Ting, R.-S. Liu, J. Am. Chem. Soc. 2006, 128, 11372–11373.
- 7P. de Frémont, N. Marion, S. P. Nolan, J. Organomet. Chem. 2009, 694, 551–560.
- 8CCDC 1502774 (2 a), 1501394 (2 o), and 1501395 (4 a) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Data Centre.
- 9K. Brand, Ber. Dtsch. Chem. Ges. 1912, 45, 3071–3077.
- 10T. Kawase, J.-i. Nishida, Chem. Rec. 2015, 15, 1045–1059.
- 11For selected contributions, see:
- 11aM. Chakraborty, C. A. Tessier, W. J. Youngs, J. Org. Chem. 1999, 64, 2947–2949;
- 11bT. Kawase, A. Konishi, Y. Hirao, K. Matsumoto, H. Kurata, T. Kubo, Chem. Eur. J. 2009, 15, 2653–2661;
- 11cT. Maekawa, Y. Segawa, K. Itami, Chem. Sci. 2013, 4, 2369–2373;
- 11dJ. Shen, D. Yuan, Y. Qiao, X. Shen, Z. Zhang, Y. Zhong, Y. Yi, X. Zhu, Org. Lett. 2014, 16, 4924–4927.
- 12
- 12aZ. U. Levi, T. D. Tilley, J. Am. Chem. Soc. 2009, 131, 2796–2797;
- 12bJ. Zhao, K. Oniwa, N. Asao, Y. Yamamoto, T. Jin, J. Am. Chem. Soc. 2013, 135, 10222–10225.
- 13
- 13aC. Chen, M. Harhausen, R. Liedtke, K. Bussmann, A. Fukazawa, S. Yamaguchi, J. L. Petersen, C. G. Daniliuc, R. Fröhlich, G. Kehr, G. Erker, Angew. Chem. Int. Ed. 2013, 52, 5992–5996; Angew. Chem. 2013, 125, 6108–6112;
- 13bC. Chen, M. Harhausen, A. Fukazawa, S. Yamaguchi, R. Fröhlich, C. G. Daniliuc, J. L. Petersen, G. Kehr, G. Erker, Chem. Asian J. 2014, 9, 1671–1681.
- 14A. S. K. Hashmi, Angew. Chem. Int. Ed. 2010, 49, 5232–5241; Angew. Chem. 2010, 122, 5360–5369.
- 15G. Schröder, J. F. M. Oth, R. Merényi, Angew. Chem. 1965, 77, 774–784.