Hollow Iron–Vanadium Composite Spheres: A Highly Efficient Iron-Based Water Oxidation Electrocatalyst without the Need for Nickel or Cobalt
Dr. Ke Fan
Department of Chemistry, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 430070 Wuhan, China
Search for more papers by this authorDr. Yongfei Ji
School of Biotechnology, KTH Royal Institute of Technology, 10691 Stockholm, Sweden
Search for more papers by this authorHaiyuan Zou
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 430070 Wuhan, China
Search for more papers by this authorDr. Jinfeng Zhang
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 430070 Wuhan, China
Search for more papers by this authorBicheng Zhu
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 430070 Wuhan, China
Search for more papers by this authorDr. Hong Chen
Department of Chemistry, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
Search for more papers by this authorQuentin Daniel
Department of Chemistry, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
Search for more papers by this authorProf. Yi Luo
School of Biotechnology, KTH Royal Institute of Technology, 10691 Stockholm, Sweden
Search for more papers by this authorCorresponding Author
Prof. Jiaguo Yu
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 430070 Wuhan, China
Search for more papers by this authorCorresponding Author
Prof. Licheng Sun
Department of Chemistry, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
State Key Lab of Fine Chemicals, DUT-KTH Joint Education and Research Center on Molecular Devices, Dalian University of Technology, 116024 Dalian, China
Search for more papers by this authorDr. Ke Fan
Department of Chemistry, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 430070 Wuhan, China
Search for more papers by this authorDr. Yongfei Ji
School of Biotechnology, KTH Royal Institute of Technology, 10691 Stockholm, Sweden
Search for more papers by this authorHaiyuan Zou
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 430070 Wuhan, China
Search for more papers by this authorDr. Jinfeng Zhang
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 430070 Wuhan, China
Search for more papers by this authorBicheng Zhu
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 430070 Wuhan, China
Search for more papers by this authorDr. Hong Chen
Department of Chemistry, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
Search for more papers by this authorQuentin Daniel
Department of Chemistry, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
Search for more papers by this authorProf. Yi Luo
School of Biotechnology, KTH Royal Institute of Technology, 10691 Stockholm, Sweden
Search for more papers by this authorCorresponding Author
Prof. Jiaguo Yu
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, 430070 Wuhan, China
Search for more papers by this authorCorresponding Author
Prof. Licheng Sun
Department of Chemistry, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
State Key Lab of Fine Chemicals, DUT-KTH Joint Education and Research Center on Molecular Devices, Dalian University of Technology, 116024 Dalian, China
Search for more papers by this authorGraphical Abstract
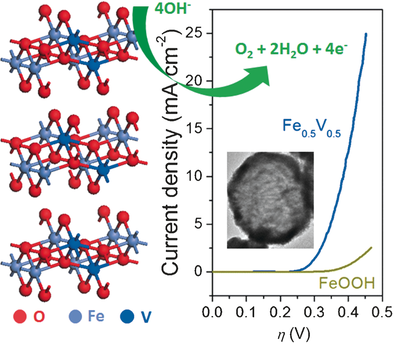
Missing, but not missed: Vanadium-doped FeOOH is a low-cost, highly efficient iron-based electrocatalyst for water oxidation without Ni or Co participation. It exhibits a low overpotential 390 mV (10 mA cm−2 catalytic current density), low Tafel slope of 36.7 mV dec−1, and a considerable durability.
Abstract
Noble-metal-free bimetal-based electrocatalysts have shown high efficiency for water oxidation. Ni and/or Co in these electrocatalysts are essential to provide a conductive, high-surface area and a chemically stable host. However, the necessity of Ni or Co limits the scope of low-cost electrocatalysts. Herein, we report a hierarchical hollow FeV composite, which is Ni- and Co-free and highly efficient for electrocatalytic water oxidation with low overpotential 390 mV (10 mA cm−2 catalytic current density), low Tafel slope of 36.7 mV dec−1, and a considerable durability. This work provides a novel and efficient catalyst, and greatly expands the scope of low-cost Fe-based electrocatalysts for water splitting without need of Ni or Co.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201611863-sup-0001-misc_information.pdf3.1 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. C. L. McCrory, S. Jung, J. C. Peters, T. F. Jaramillo, J. Am. Chem. Soc. 2013, 135, 16977–16987.
- 2
- 2aJ. R. Galán-Mascarós, ChemElectroChem 2015, 2, 37–50;
- 2bR. D. L. Smith, M. S. Prévot, R. D. Fagan, Z. Zhang, P. A. Sedach, M. K. J. Siu, S. Trudel, C. P. Berlinguette, Science 2013, 340, 60–63.
- 3
- 3aX. Yu, M. Zhang, W. Yuan, G. Shi, J. Mater. Chem. A 2015, 3, 6921–6928;
- 3bJ. Nai, H. Yin, T. You, L. Zheng, J. Zhang, P. Wang, Z. Jin, Y. Tian, J. Liu, Z. Tang, L. Guo, Adv. Energy Mater. 2015, 5, 1401880;
- 3cS. Chen, J. Duan, M. Jaroniec, S. Z. Qiao, Angew. Chem. Int. Ed. 2013, 52, 13567–13570; Angew. Chem. 2013, 125, 13812–13815;
- 3dX. Long, J. Li, S. Xiao, K. Yan, Z. Wang, H. Chen, S. Yang, Angew. Chem. Int. Ed. 2014, 53, 7584–7588; Angew. Chem. 2014, 126, 7714–7718.
- 4
- 4aF. Song, X. Hu, Nat. Commun. 2014, 5, 4477;
- 4bS. J. Kim, Y. Lee, D. K. Lee, J. W. Lee, J. K. Kang, J. Mater. Chem. A 2014, 2, 4136–4139;
- 4cJ. Wang, W. Cui, Q. Liu, Z. Xing, A. M. Asiri, X. Sun, Adv. Mater. 2016, 28, 215–230.
- 5C. C. L. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman, J. C. Peters, T. F. Jaramillo, J. Am. Chem. Soc. 2015, 137, 4347–4357.
- 6L. Han, S. Dong, E. Wang, Adv. Mater. 2016, 28, 9266–9291.
- 7
- 7aC. Long, L. Jiang, T. Wei, J. Yan, Z. Fan, J. Mater. Chem. A 2014, 2, 16678–16686;
- 7bH. Abdel-Samad, P. R. Watson, Appl. Surf. Sci. 1998, 136, 46–54.
- 8N. S. McIntyre, D. G. Zetaruk, Anal. Chem. 1977, 49, 1521–1529.
- 9
- 9aG. Silversmit, D. Depla, H. Poelman, G. B. Marin, R. De Gryse, J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175;
- 9bK. Fan, H. Chen, Y. Ji, H. Huang, P. M. Claesson, Q. Daniel, B. Philippe, H. Rensmo, F. Li, Y. Luo, L. Sun, Nat. Commun. 2016, 7, 11981.
- 10U. Schwertmann, G. Pfab, Geochim. Cosmochim. Acta 1994, 58, 4349–4352.
- 11
- 11aG. K. Schweitzer, L. L. Pesterfield, The Aqueous Chemistry of the Elements, Oxford University Press, Oxford, 2010;
10.1093/oso/9780195393354.001.0001 Google Scholar
- 11bM. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith, S. W. Boettcher, J. Am. Chem. Soc. 2015, 137, 3638–3648;
- 11cM. S. Burke, S. Zou, L. J. Enman, J. E. Kellon, C. A. Gabor, E. Pledger, S. W. Boettcher, J. Phys. Chem. Lett. 2015, 6, 3737–3742.
- 12T. W. Kim, K.-S. Choi, Science 2014, 343, 990–994.
- 13C. Wu, X. Zhang, B. Ning, J. Yang, Y. Xie, Inorg. Chem. 2009, 48, 6044–6054.
- 14
- 14aC. Qiao, Y. Zhang, Y. Zhu, C. Cao, X. Bao, J. Xu, J. Mater. Chem. A 2015, 3, 6878–6883;
- 14bY. Li, L. Zhang, X. Xiang, D. Yan, F. Li, J. Mater. Chem. A 2014, 2, 13250–13258.