Synthesis of Enantiopure C3-Symmetric Triangular Molecules
Corresponding Author
Dr. Tomoya Miura
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto, 615-8510 Japan
Search for more papers by this authorTakayuki Nakamuro
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto, 615-8510 Japan
Search for more papers by this authorDr. Scott G. Stewart
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto, 615-8510 Japan
School of Chemistry and Biochemistry, The University of Western Australia, Australia
Search for more papers by this authorDr. Yuuya Nagata
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto, 615-8510 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Masahiro Murakami
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto, 615-8510 Japan
Search for more papers by this authorCorresponding Author
Dr. Tomoya Miura
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto, 615-8510 Japan
Search for more papers by this authorTakayuki Nakamuro
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto, 615-8510 Japan
Search for more papers by this authorDr. Scott G. Stewart
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto, 615-8510 Japan
School of Chemistry and Biochemistry, The University of Western Australia, Australia
Search for more papers by this authorDr. Yuuya Nagata
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto, 615-8510 Japan
Search for more papers by this authorCorresponding Author
Prof. Dr. Masahiro Murakami
Department of Synthetic Chemistry and Biological Chemistry, Kyoto University, Katsura, Kyoto, 615-8510 Japan
Search for more papers by this authorGraphical Abstract
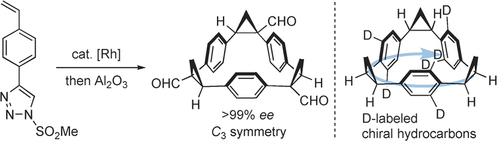
My hat, it has three corners: Enantiopure C3-symmetric triangular macrocycles were synthesized by means of triple asymmetric cyclopropanation. This method is successfully extended to the synthesis of an enantiopure hydrocarbon, which owes its chirality to asymmetric distribution of H/D atoms on the benzene rings.
Abstract
An asymmetric synthesis of C3-symmetric triangular macrocycles is reported. 1-Methylsulfonyl-4-(4-vinylphenyl)-1,2,3-triazole undergoes a rhodium(II)-catalyzed cyclotrimerization to establish an enantiopure C3-symmetric triangular macrocycle motif. This method can be applied to the synthesis of an enantiopure hydrocarbon, which owes its chirality to asymmetric distribution of H/D atoms on the benzene rings.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie201612585-sup-0001-misc_information.pdf12 MB | Supplementary |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. Moberg, Angew. Chem. Int. Ed. 1998, 37, 248;
10.1002/(SICI)1521-3773(19980216)37:3<248::AID-ANIE248>3.0.CO;2-5 CAS PubMed Web of Science® Google ScholarAngew. Chem. 1998, 110, 260;
- 1bS. E. Gibson, M. P. Castaldi, Angew. Chem. Int. Ed. 2006, 45, 4718; Angew. Chem. 2006, 118, 4834;
- 1cL. H. Gade, S. Bellemin-Laponnaz, Chem. Eur. J. 2008, 14, 4142;
- 1dA. Szumna, Chem. Soc. Rev. 2010, 39, 4274;
- 1eC. Moberg, Isr. J. Chem. 2012, 52, 653.
- 2Tribenzotriquinacene:
- 2aG. Markopoulos, L. Henneicke, J. Shen, Y. Okamoto, P. G. Jones, H. Hopf, Angew. Chem. Int. Ed. 2012, 51, 12884; Angew. Chem. 2012, 124, 13057;
- 2bB. Bredenkötter, M. Grzywa, M. Alaghemandi, R. Schmid, W. Herrebout, P. Bultinck, D. Volkmer, Chem. Eur. J. 2014, 20, 9100;
- 2cW. Greschner, B. Neumann, H.-G. Stammler, H. Gröger, D. Kuck, Angew. Chem. Int. Ed. 2015, 54, 13764; Angew. Chem. 2015, 127, 13968;
- 2dD. Beaudoin, F. Rominger, M. Mastalerz, Eur. J. Org. Chem. 2016, 4470.
- 3Cyclotriveratrylene:
- 3aJ. Canceill, A. Collet, J. Gabard, G. Gottarelli, G. P. Spada, J. Am. Chem. Soc. 1985, 107, 1299;
- 3bZ. Luz, R. Poupko, E. J. Wachtel, H. Zheng, N. Friedman, X. Cao, T. B. Freedman, L. A. Nafie, H. Zimmermann, J. Phys. Chem. A 2007, 111, 10507;
- 3cJ.-T. Yu, Z.-T. Huang, Q.-Y. Zheng, Org. Biomol. Chem. 2012, 10, 1359.
- 4Trioxatricornan:
- 4aS. K. Narasimhan, D. J. Kerwood, L. Wu, J. Li, R. Lombardi, T. B. Freedman, Y.-Y. Luk, J. Org. Chem. 2009, 74, 7023. See also
- 4bP. Mobian, C. Nicolas, E. Francotte, T. Bürgi, J. Lacour, J. Am. Chem. Soc. 2008, 130, 6507;
- 4cM. Yamamura, T. Saito, T. Nabeshima, J. Am. Chem. Soc. 2014, 136, 14299.
- 5Subphthalocyanine: S. Shimizu, A. Miura, S. Khene, T. Nyokong, N. Kobayashi, J. Am. Chem. Soc. 2011, 133, 17322.
- 6Others:
- 6aS. Higashibayashi, H. Sakurai, J. Am. Chem. Soc. 2008, 130, 8592;
- 6bN. Saito, R. Terakawa, M. Yamaguchi, Chem. Eur. J. 2014, 20, 5601;
- 6cG.-W. Zhang, P.-F. Li, Z. Meng, H.-X. Wang, Y. Han, C.-F. Chen, Angew. Chem. Int. Ed. 2016, 55, 5304; Angew. Chem. 2016, 128, 5390.
- 7anti-[2]CPPC: E. A. Truesdale, R. S. Hutton, J. Am. Chem. Soc. 1979, 101, 6475.
- 8[n]Cycloparaphenyleneacetylene:
- 8aT. Kawase, H. R. Darabi, M. Oda, Angew. Chem. Int. Ed. Engl. 1996, 35, 2664; Angew. Chem. 1996, 108, 2803;
- 8bK. Miki, T. Matsushita, Y. Inoue, Y. Senda, T. Kowada, K. Ohe, Chem. Commun. 2013, 49, 9092;
- 8cS. Lee, E. Chénard, D. L. Gray, J. S. Moore, J. Am. Chem. Soc. 2016, 138, 13814.
- 9[n]Cycloparaphenylene:
- 9aR. Jasti, J. Bhattacharjee, J. B. Neaton, C. R. Bertozzi, J. Am. Chem. Soc. 2008, 130, 17646;
- 9bH. Takaba, H. Omachi, Y. Yamamoto, J. Bouffard, K. Itami, Angew. Chem. Int. Ed. 2009, 48, 6112; Angew. Chem. 2009, 121, 6228;
- 9cS. Yamago, Y. Watanabe, T. Iwamoto, Angew. Chem. Int. Ed. 2010, 49, 757; Angew. Chem. 2010, 122, 769.
- 10
- 10aY. Fujioka, Bull. Chem. Soc. Jpn. 1984, 57, 3494;
- 10bT. Shibata, M. Fujimoto, H. Hirashima, T. Chiba, K. Endo, Synthesis 2012, 44, 3269. See also
- 10cK. Tahara, T. Fujita, M. Sonoda, M. Shiro, Y. Tobe, J. Am. Chem. Soc. 2008, 130, 14339.
- 11A. de Meijere, S. I. Kozhushkov, H. Schill, Chem. Rev. 2006, 106, 4926.
- 12S. Chuprakov, S. W. Kwok, L. Zhang, L. Lercher, V. V. Fokin, J. Am. Chem. Soc. 2009, 131, 18034.
- 13(S)-NTTL=N-naphthoyl-(S)-tert-leucinate. P. Müller, Y. Allenbach, E. Robert, Tetrahedron: Asymmetry 2003, 14, 779.
- 14Initiative examples on the synthetic transformation of triazoles with extrusion of molecular nitrogen:
- 14aI. Bae, H. Han, S. Chang, J. Am. Chem. Soc. 2005, 127, 2038;
- 14bT. Horneff, S. Chuprakov, N. Chernyak, V. Gevorgyan, V. V. Fokin, J. Am. Chem. Soc. 2008, 130, 14972;
- 14cT. Miura, M. Yamauchi, M. Murakami, Chem. Commun. 2009, 1470; A recent review:
- 14dY. Jiang, R. Sun, X.-Y. Tang, M. Shi, Chem. Eur. J. 2016, 22, 17910. Selected recent papers:
- 14eJ. H. Kim, T. Gensch, D. Zhao, L. Stegemann, C. A. Strassert, F. Glorius, Angew. Chem. Int. Ed. 2015, 54, 10975; Angew. Chem. 2015, 127, 11126;
- 14fD. J. Lee, D. Ko, E. J. Yoo, Angew. Chem. Int. Ed. 2015, 54, 13715; Angew. Chem. 2015, 127, 13919;
- 14gV. N. G. Lindsay, H. M.-F. Viart, R. Sarpong, J. Am. Chem. Soc. 2015, 137, 8368;
- 14hJ. He, Y. Shi, W. Cheng, Z. Man, D. Yang, C.-Y. Li, Angew. Chem. Int. Ed. 2016, 55, 4557; Angew. Chem. 2016, 128, 4633.
- 15J. Raushel, V. V. Fokin, Org. Lett. 2010, 12, 4952.
- 16A. Boyer, Org. Lett. 2014, 16, 5878.
- 17The tetramer 4 is diastereomerically pure but awaits full characterization.
- 18CCDC 1522059 (3) and 1522058 (11) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre.
- 19H. M. Walborsky, L. E. Allen, J. Am. Chem. Soc. 1971, 93, 5465.
- 20
- 20aJ. Haesler, I. Schindelholz, E. Riguet, C. G. Bochet, W. Hug, Nature 2007, 446, 526;
- 20bA. Masarwa, D. Gerbig, L. Oskar, A. Loewenstein, H. P. Reisenauer, P. Lesot, P. R. Schreiner, I. Marek, Angew. Chem. Int. Ed. 2015, 54, 13106; Angew. Chem. 2015, 127, 13298.