Journal list menu
Export Citations
Download PDFs
Cover Pictures
Cover Picture: A Suite of Solid-State NMR Experiments for RNA Intranucleotide Resonance Assignment in a 21 kDa Protein–RNA Complex (Angew. Chem. Int. Ed. 38/2013)
- Page: 9861
- First Published: 22 August 2013

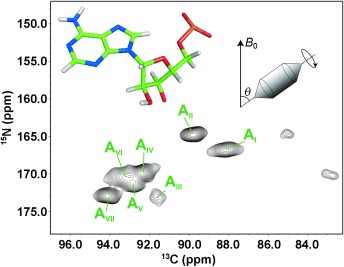
Resonance assignment is the first step of RNA structure determination by NMR spectroscopy. In their Communication on page 9996 ff., T. Carlomagno and co-workers present a suite of solid-state NMR experiments for RNA intranucleotide resonance assignment. These experiments open the way to structural studies of RNA by solid-state NMR spectroscopy.
Inside Cover: Simultaneous Self-Assembly of a [2]Catenane, a Trefoil Knot, and a Solomon Link from a Simple Pair of Ligands (Angew. Chem. Int. Ed. 38/2013)
- Page: 9862
- First Published: 02 August 2013
![Inside Cover: Simultaneous Self-Assembly of a [2]Catenane, a Trefoil Knot, and a Solomon Link from a Simple Pair of Ligands (Angew. Chem. Int. Ed. 38/2013)](/cms/asset/bfd581c7-2f2b-4b2b-894f-a4442ecc14ca/mcontent.jpg)
Three molecular links were prepared in one pot from a single pair of chelating ligands. In their Communication on page 9956 ff., L. J. Charbonnière, A. Trabolsi et al. describe the self-assembly of a [2]catenane, a trefoil knot, and a Solomon link through combined metal templation, dynamic covalent-bond formation, and noncovalent interactions. During the reaction, product evolution was monitored by mass spectrometry, which provided evidence for the mechanistic routes to all three products.
Inside Back Cover: Catalysis with Anion–π Interactions (Angew. Chem. Int. Ed. 38/2013)
- Page: 10125
- First Published: 26 August 2013

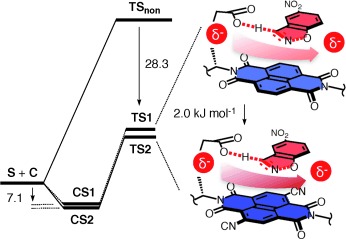
The increasing stabilization of an anionic transition state with the increasing π acidity of newly made catalysts provides unprecedented experimental evidence that anion–π interactions can contribute to catalysis. In their Communication on page 9940 ff., S. Matile et al. further disclose theoretical simulations of anion–π catalysis that illustrate how the negative charge slides over the π-acidic surface of the naphthalenediimide catalyst.
Back Cover: Dynamic Single Crystals: Kinematic Analysis of Photoinduced Crystal Jumping (The Photosalient Effect) (Angew. Chem. Int. Ed. 38/2013)
- Page: 10126
- First Published: 19 August 2013

When subjected to a light stimulus crystals of certain materials can suddenly hop and leap over distances 102–105 times their own size to release the strain that accumulates in their interior as a result of a photochemical reaction. This photosalient effect is an impressive demonstration of the rapid conversion of light into mechanical motion on a macroscopic scale. In their Communication on page 9990 ff., P. Naumov, E. V. Boldyreva, and co-workers analyze the kinematic motion patterns. Picture design by Bailey Curzadd.
Editorial
Editorial: Probes, Sensors, and Labels: Why is Real Progress Slow?
- Pages: 9864-9865
- First Published: 28 August 2013
Graphical Abstract
Graphical Abstract: Angew. Chem. Int. Ed. 38/2013
- Pages: 9867-9881
- First Published: 12 September 2013
News
Spotlights on our sister journals: Angew. Chem. Int. Ed. 38/2013
- Pages: 9882-9885
- First Published: 12 September 2013
Author Profile
News
Book Review
Design and Applications of Single-Site Heterogeneous Catalysts. Contributions to Green Chemistry, Clean Technology and Sustainability. By John Meurig Thomas.
- Pages: 9892-9893
- First Published: 21 August 2013
Highlight
CH Activation
8-Aminoquinoline: A Powerful Directing Group in Metal-Catalyzed Direct Functionalization of CH Bonds
- Pages: 9896-9898
- First Published: 12 August 2013
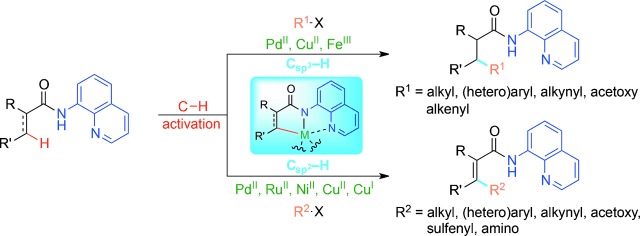
Chelate me if you can: Over the last decade, strategies for the functionalization of both C H and C
H and C H bonds have witnessed an increasing use of a simple, yet powerful directing group, 8-aminoquinoline (in blue). This auxiliary is very efficient in a wide range of metal-mediated reactions, and can be readily removed to afford the desired carboxylic acids or corresponding derivatives.
H bonds have witnessed an increasing use of a simple, yet powerful directing group, 8-aminoquinoline (in blue). This auxiliary is very efficient in a wide range of metal-mediated reactions, and can be readily removed to afford the desired carboxylic acids or corresponding derivatives.
Review
Aromatic Dehydrogenation
Comparison of Oxidative Aromatic Coupling and the Scholl Reaction
- Pages: 9900-9930
- First Published: 14 July 2013
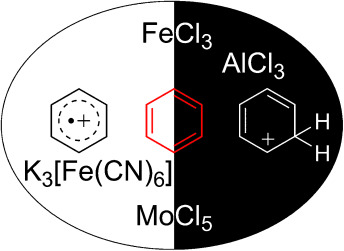
Where is the oxidant? Although the coupling of arenes in the presence of Lewis acids has been known for some time, the differentiation between reactions in the presence of a non-oxidizing Lewis acid (Scholl reaction) and in the presence of an oxidizing Lewis acid has been lost over the years. This Review highlights the similarities and differences between these reactions, which most probably proceed by two different mechanisms.
Communications
Nanoscale Polymetallic Assemblies
A Ring of Rings and Other Multicomponent Assemblies of Cages†
- Pages: 9932-9935
- First Published: 31 July 2013
Fluorescent Probes
Lipid Pools As Photolabile “Protecting Groups”: Design of Light-Activatable Bioagents†
- Pages: 9936-9939
- First Published: 31 July 2013
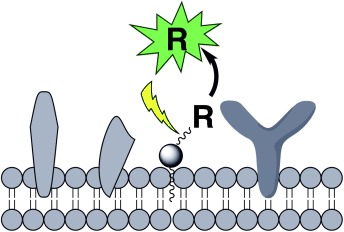
Inactive in the membrane: Lipidated light-responsive constructs that sequester bioagents (R, see scheme) to the membranes of organelles and cells have been constructed. When membrane-bound, the bioagent is not susceptible to processing by its biological target. Photolysis releases the bioagent from its membrane anchor and thereby renders it biologically active.
Anion–π Interactions
Thiodepsipeptides
A High-Resolution Structure that Provides Insight into Coiled-Coil Thiodepsipeptide Dynamic Chemistry†
- Pages: 9944-9947
- First Published: 08 August 2013
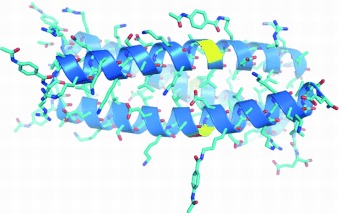
Stable and reactive: A crystal structure at 1.35 Å of a thioester coiled-coil protein reveals high similarity to all-peptide-bond proteins. In these assemblies, the thioester bonds are kept reactive towards thiol molecules in the mixture. This enables efficient domain exchange between proteins in response to changes in folding conditions or introduction of external templates.
Shape Transformation
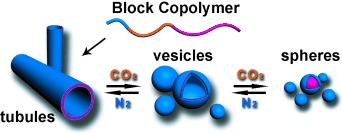
Polymeric Microtubules That Breathe: CO2-Driven Polymer Controlled-Self-Assembly and Shape Transformation†
- Pages: 9948-9951
- First Published: 08 August 2013
Au Nanosheets
Self-Assembly of Au15 into Single-Cluster-Thick Sheets at the Interface of Two Miscible High-Boiling Solvents†
- Pages: 9952-9955
- First Published: 27 August 2013
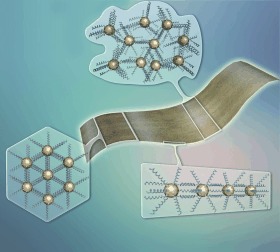
Wet (nano)blanket: The self-assembly of Au nanoclusters into single-cluster-thick nanosheets is performed in two miscible high-boiling solvents with a slight polarity difference, which generates microphase separation and acts as a soft template to direct the self-assembly in a two-dimensional orientation.
Molecular Knots
Simultaneous Self-Assembly of a [2]Catenane, a Trefoil Knot, and a Solomon Link from a Simple Pair of Ligands†
- Pages: 9956-9960
- First Published: 05 July 2013
Photonic Hydrogels
Polymerized Microgel Colloidal Crystals: Photonic Hydrogels with Tunable Band Gaps and Fast Response Rates†
- Pages: 9961-9965
- First Published: 08 August 2013

Into and out of the blue: The highly ordered structure of a PNIPAM microgel colloidal crystal (MCC) is stabilized by photopolymerization of its surface-bound vinyl groups. The resulting polymerized MCCs can respond reversibly and quickly to external stimuli, including temperature and ionic strength of the surrounding media, allowing the color (see picture) and band gap to be finely tuned in the whole visible range.
B,N Heterocycles
BN-Dibenzo[a,o]picenes: Analogues of an Unknown Polycyclic Aromatic Hydrocarbon†
- Pages: 9966-9969
- First Published: 29 July 2013
![BN-Dibenzo[a,o]picenes: Analogues of an Unknown Polycyclic Aromatic Hydrocarbon](/cms/asset/b1659a17-fb32-4312-9070-f1bc44e0def0/mcontent.jpg)
Reflecting on synthetic pinnacles: Whereas the parent hydrocarbon is not readily accessible, several examples of BN substituted-dibenzo[a,o]picenes can be prepared in two steps from known starting materials. These non-linear heptacene analogues are water-stable materials. Their preparation utilizes a potentially general method for preparing extended BN analogues of difficult-to-synthesize polycyclic aromatic hydrocarbon frameworks.
Helical Structures
Rapid Access to Dibenzohelicenes and their Functionalized Derivatives†
- Pages: 9970-9975
- First Published: 09 August 2013
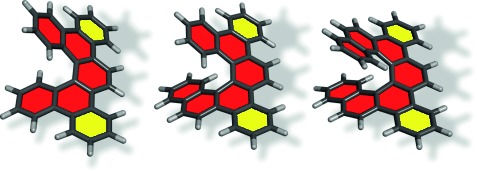
Spiraling up: Easy access to dibenzo[5]-, dibenzo[6]-, and dibenzo[7]helicenes (see picture) as well as their functionalized derivatives includes Sonogashira and Suzuki–Miyaura couplings, desilylation, and [2+2+2] alkyne cycloisomerization. The simplicity of this non-photochemical approach combined with the potential for helicity control favors dibenzohelicenes over the parent helicenes for practical applications.
Synthetic Riboswitches
A Synthetic Riboswitch that Operates using a Rationally Designed Ligand–RNA Pair†
- Pages: 9976-9979
- First Published: 03 September 2013
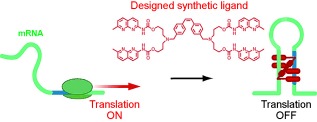
The construction of an artificial riboswitch is based on a ligand–RNA pair without any molecular biology-based selection processes. The ligand selectively and significantly stabilized an RNA duplex containing an r(XGG)/r(XGG) sequence (X=U, A, G). The integration of the ligand-binding sequences into the 5′-untranslated region of mRNA provided an artificial riboswitch that was responsive to Z-NCTS.
Alkaloid Biosynthesis
Discovery of McbB, an Enzyme Catalyzing the β-Carboline Skeleton Construction in the Marinacarboline Biosynthetic Pathway†
- Pages: 9980-9984
- First Published: 01 August 2013
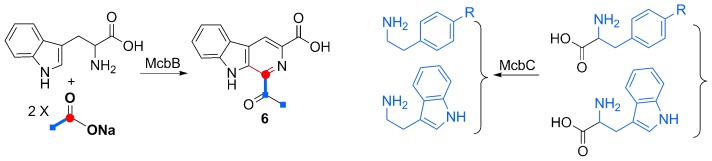
Three genes, mcbABC, that drive the biosynthesis of marinacarbolines, have been elucidated through genome mining, gene inactivation, heterologous expression, feeding, and site-directed mutagenesis experiments. McbB is highlighted as a novel enzyme for the β-carboline core construction, which involves a Pictet–Spengler cyclization process and requiring E97 for biochemical activity.
Zeolite Catalysis
A Common Intermediate for N2 Formation in Enzymes and Zeolites: Side-On Cu–Nitrosyl Complexes†
- Pages: 9985-9989
- First Published: 12 August 2013
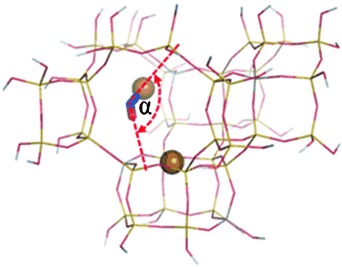
Side on! Combined FTIR and NMR studies revealed the presence of a side-on nitrosyl species in the zeolite Cu-SSZ-13. This intermediate is very similar to those found in nitrite reductase enzyme systems. The identification of this intermediate led to the proposal of a reaction mechanism that is fully consistent with the results of both kinetic and spectroscopic studies.
Actuators
Dynamic Single Crystals: Kinematic Analysis of Photoinduced Crystal Jumping (The Photosalient Effect)†
- Pages: 9990-9995
- First Published: 19 July 2013
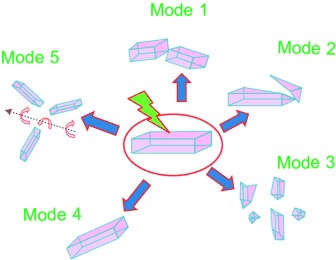
Crystals on the move: If they are subjected to a strong light stimulus, crystals of the cobalt coordination compound [Co(NH3)5(NO2)]Cl(NO3) undergo sudden jumps and leap over distances 102–105 times their own size to release the strain that accumulates in their interior. The first quantitative kinematic analysis of this phenomenon is reported. The observed effect could be employed for actuation on the macroscopic scale.
Protein–RNA Complexes
A Suite of Solid-State NMR Experiments for RNA Intranucleotide Resonance Assignment in a 21 kDa Protein–RNA Complex†
- Pages: 9996-10001
- First Published: 26 July 2013
NMR Spectroscopy
Molecular Mechanism of Prion Protein Oligomerization at Atomic Resolution†
- Pages: 10002-10005
- First Published: 09 August 2013
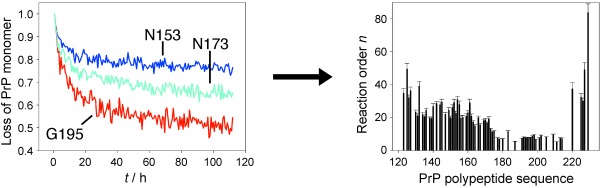
Prion protein oligomerization: Despite the crucial role of oligomers during prion protein (PrP) pathogenesis the molecular mechanism of their formation has remained largely elusive. A 2D time-resolved NMR study which made it possible to characterize the oligomerization kinetics with unprecedented site-specificity is reported (see picture).
Drug Discovery
Steering Target Selectivity and Potency by Fragment-Based De Novo Drug Design†
- Pages: 10006-10009
- First Published: 26 August 2013
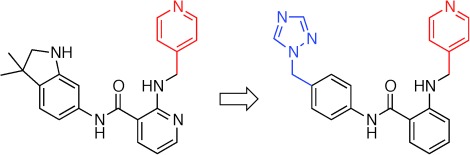
Kinase inhibitors: Ligand-based de novo design is validated as a viable technology for rapidly generating innovative compounds possessing the desired biochemical profile. The study discloses the discovery of the most selective vascular endothelial growth factor receptor-2 (VEGFR-2) kinase inhibitor (right in scheme) known to date as prime lead for antiangiogenic drug development.
RNA Targeting
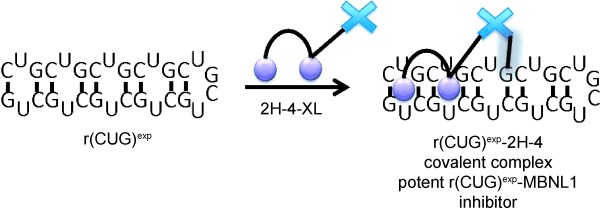
Covalent Small-Molecule–RNA Complex Formation Enables Cellular Profiling of Small-Molecule–RNA Interactions†
- Pages: 10010-10013
- First Published: 01 August 2013
Nanoparticles
Capping with Multivalent Surfactants for Zeolite Nanocrystal Synthesis†
- Pages: 10014-10017
- First Published: 01 August 2013
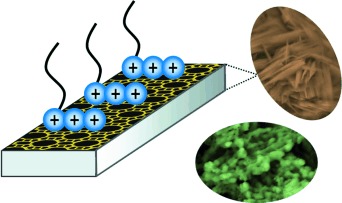
Multiammonium surfactants exhibited a remarkable capping effect for zeolite synthesis in the forms of nanoparticles, nanorods, and nanosponges in cases where common monovalent surfactants failed. A nanorod-shaped mordenite zeolite synthesized in this manner showed significantly enhanced catalytic lifetimes in acid-catalyzed cumene synthesis reactions.
Fluorescent Probes
A Unique Family of Rigid Analogues of the GFP Chromophore with Tunable Two-Photon Action Cross-Sections for Biological Imaging†
- Pages: 10018-10022
- First Published: 08 August 2013
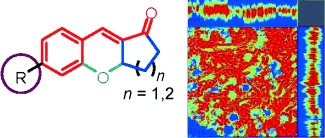
Conformationally restricted analogues of the GFP chromophore have been synthesized. The spectroscopic properties of GCTPOC, a unique family with tunable two-photon action cross-sections, were investigated. GCTPOC could be used as a robust two-photon platform for development of a two-photon fluorescent thiol sensor, which could stain endogenous thiols both in vitro and in vivo.
Synthetic Methods
Rhodium-Catalyzed Oxygenative Addition to Terminal Alkynes for the Synthesis of Esters, Amides, and Carboxylic Acids†
- Pages: 10023-10026
- First Published: 12 August 2013

A gem of a couple: The title reaction of terminal alkynes with O and N nucleophiles proceeds in the presence of [{Rh(cod)Cl}2], P(4-FC6H4)3, and 4-picoline N-oxide. Alcohols, amines, and water add to the terminal alkynes to give esters, amides, and carboxylic acids, respectively. The reaction involves formation of a rhodium vinylidene, oxidation to a ketene by oxygen transfer, and nucleophilic addition.
Polymerization
Visible-Light Hypervalent Iodide Carboxylate Photo(trifluoro)methylations and Controlled Radical Polymerization of Fluorinated Alkenes†
- Pages: 10027-10030
- First Published: 12 August 2013
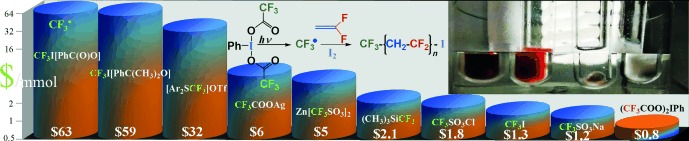
IFAB-ulous trifluoromethylation: (CX3COO)2IIIIPh (X=F, H) and (CH3COO)3IV(C6H4COO) are introduced as CX3./CX3I precursors for metal-free, visible-light, radical (trifluoro)(iodo)methylations of alkenes, illustrated by their use as photoinitiators for the controlled radical polymerization of vinylidene fluoride with external (I(CF2)6I) and in situ generated (CF3I) iodine chain transfer agents, and for block copolymer synthesis.
Organoaluminum Compounds
Syntheses and Structures of an “Alumole” and Its Dianion†
- Pages: 10031-10034
- First Published: 08 August 2013
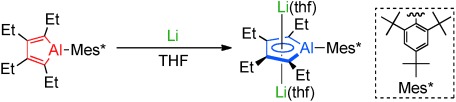
Base free: An alumole was synthesized and treatment with lithium afforded the lithium salt of the alumole dianion. The structures of these two molecules were then investigated. The CC bond lengths of the AlC4 ring in the dianion are nearly equal. DFT calculations revealed that the 3p(Al)–π* conjugation lowers the LUMO level of the alumole and that coordination of two lithium cations to the alumole dianion results in a planar AlC4 ring.
Cross-Coupling
A General, Practical Palladium-Catalyzed Cyanation of (Hetero)Aryl Chlorides and Bromides†
- Pages: 10035-10039
- First Published: 09 August 2013
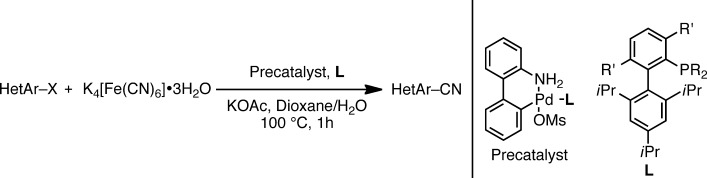
Playing it safe: The nontoxic cyanide source K4[Fe(CN)6]⋅3 H2O can be used for the cyanation of (hetero)aryl halides. The application of palladacycle catalysts prevents poisoning during catalyst formation, thereby allowing for low catalyst loadings, fast reaction times, and wide heterocyclic substrate scope.
Synthetic Methods
Transition-Metal-Free Multicomponent Reactions Involving Arynes, N-Heterocycles, and Isatins†
- Pages: 10040-10043
- First Published: 01 August 2013
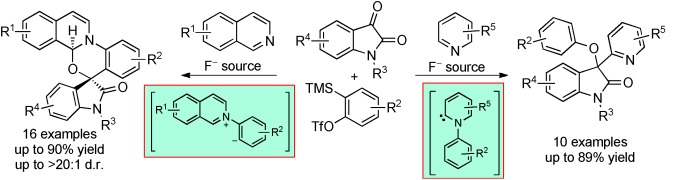
Mix and match: With isoquinoline as the nucleophilic trigger, multicomponent reactions afforded spirooxazino isoquinoline derivatives, proceeding through 1,4-dipolar intermediates. The use of pyridine as a nucleophile furnished indolin-2-one derivatives, with the reaction likely proceeding through a pyridylidene intermediate.
Asymmetric Catalysis
Rhodium-Catalyzed Tandem Cyclopropanation/Cope Rearrangement of 4-Alkenyl-1-sulfonyl-1,2,3-triazoles with Dienes†
- Pages: 10044-10047
- First Published: 01 August 2013
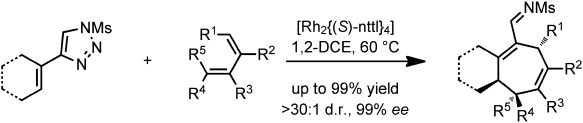
Take your pick…︁ A practical method for the synthesis of structurally diverse rhodium vinylcarbenes from stable 1-sulfonyl-1,2,3-triazole precursors has been developed. The reaction is general for a broad range of 4-alkenyl triazoles and dienes, enabling the stereoselective synthesis of a variety of polycyclic imines, which are readily converted into amines or aldehydes in a one-pot process.
CH Activation
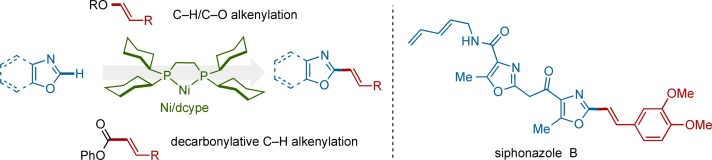
CH Alkenylation of Azoles with Enols and Esters by Nickel Catalysis†
- Pages: 10048-10051
- First Published: 19 July 2013
Natural Product Synthesis
A Synthesis of the Chlorosulfolipid Mytilipin A via a Longest Linear Sequence of Seven Steps†
- Pages: 10052-10055
- First Published: 08 August 2013
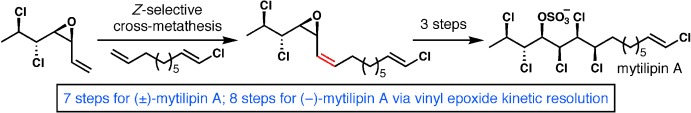
Magnificent seven: The chlorosulfolipid mytilipin A was synthesized in racemic form in seven steps and in enantioenriched form in eight steps. Key transformations include a highly diastereoselective bromoallylation of a sensitive α,β-dichloroaldehyde, a kinetic resolution of a vinyl epoxide, a convergent and highly Z-selective alkene cross-metathesis, and a chemoselective and diastereoselective dichlorination of a complex diene.
Asymmetric Catalysis
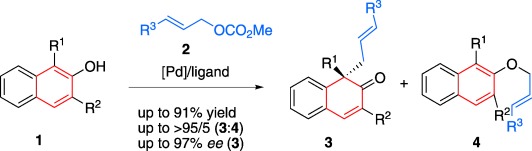
Palladium-Catalyzed Intermolecular Asymmetric Allylic Dearomatization Reaction of Naphthol Derivatives†
- Pages: 10056-10059
- First Published: 09 August 2013
Synthetic Methods
A Rapid Route to Aminocyclopropanes via Carbamatoorganozinc Carbenoids†
- Pages: 10060-10063
- First Published: 01 August 2013
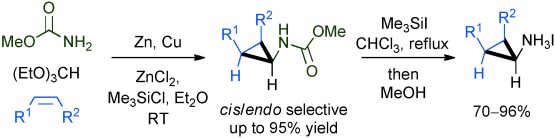
Easy as 1,2,3: Reaction of methyl carbamate, triethyl orthoformate, and readily available alkenes provides a highly practical preparation of protected aminocyclopropanes. The reaction proceeds with preferential cis addition to alkenes, and cleavage of the methyl carbamate gives the free aminocyclopropanes as their HI salts (see scheme).
Organophosphorus Compounds
The 2-Phosphaethynolate Anion: A Convenient Synthesis and [2+2] Cycloaddition Chemistry†
- Pages: 10064-10067
- First Published: 01 August 2013
![The 2-Phosphaethynolate Anion: A Convenient Synthesis and [2+2] Cycloaddition Chemistry](/cms/asset/3bd014fc-6df7-487e-93d5-d7a20b9de2eb/mcontent.jpg)
Hip to be square: Direct carbonylation of solutions of the heptaphosphide trianion (P73−) afforded the phosphaethynolate anion in moderate yields. This species undergoes [2+2] cycloaddition reactions with diphenylketene and bis(2,6-diisopropylphenyl)carbodiimide to yield the anionic four-membered heterocycles P[C(O)]2C(C6H5)2− and PC(O)(CNDipp)NDipp−.
Metallabenzenes
Building a Parent Iridabenzene Structure from Acetylene and Dichloromethane on an Iridium Center†
- Pages: 10068-10071
- First Published: 09 August 2013
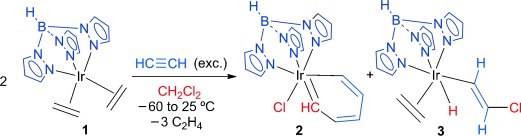
Parenthood: The reaction of [TpIr(C2H4)2] (1) (Tp=hydrotris(pyrazolyl)borate) with acetylene in CH2Cl2 affords a 1:1 mixture of the “parent” metallabenzene 2 (that is, all the ring carbon centers are CH units) and the β-Cl substituted vinyl species 3. Generation of 2 is by the coupling of an iridacyclopentadiene (formed from two acetylene molecules at the Ir center) with the dichloromethane-derived chlorocarbene “:C(H)Cl” and a subsequent α-Cl elimination event.
Natural Products
Total Syntheses of Amphidinolides T1, T3, and T4†
- Pages: 10072-10075
- First Published: 29 July 2013
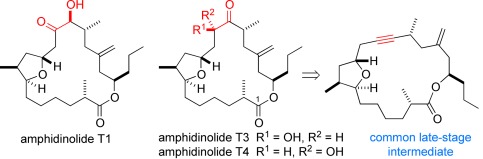
Concise and high-yielding total syntheses of amphidinolides T1, T3, and T4 have been completed using an alkynyl macrolactone as a common late-stage intermediate. The required α-hydroxy ketone motif was installed by sequential alkyne hydrosilylation, epoxidation, and Fleming–Tamao oxidation. An oxonium ylide rearrangement formed the trisubstituted tetrahydrofuran core found in the natural products.
Partial Reduction
Catalytic 1,4-Selective Hydrosilylation of Pyridines and Benzannulated Congeners†
- Pages: 10076-10079
- First Published: 01 August 2013
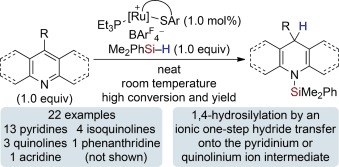
Radically different! The hydrosilylation of pyridines and quinolines is strictly 1,4-selective and likely involves an ionic one-step rather than the established radical two-step hydride transfer from a ruthenium(II) hydride complex onto the respective pyridinium and quinolinium ion intermediates (see scheme; ArF=3,5-(CF3)2C6H3). Even 4-substituted substrates react highly regioselectively. Isoquinolines yield the 1,2-reduced heterocycles.
Abnormal N-Heterocyclic Carbenes
Synthesis of an Imidazolium Phosphanide Zwitterion and Its Conversion into Anionic Imidazol-2-ylidene Derivatives†
- Pages: 10080-10083
- First Published: 08 August 2013
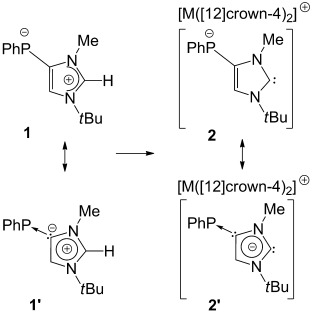
Matter of opinion: The novel zwitterion 1 has been synthesized and studied theoretically and also converted into anionic NHCs 2. The former can also be described as a phosphinidene adduct of an abnormal N-heterocyclic carbene (1′) and, in the same vein, the latter represents a phosphinidene adduct of an anionic N-heterocyclic dicarbene (2′).
Azaindole Synthesis
Preparation of Functionalized Indoles and Azaindoles by the Intramolecular Copper-Mediated Carbomagnesiation of Ynamides†
- Pages: 10084-10088
- First Published: 09 August 2013
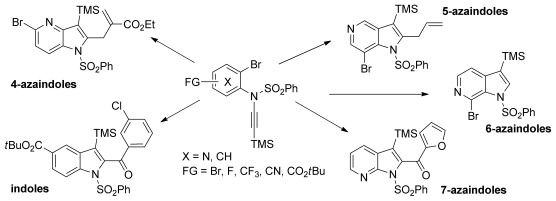
Variations on a theme: A mild and general intramolecular copper-mediated carbomagnesiation procedure for the synthesis of functionalized indoles as well as 4-, 5-, 6-, and 7-azaindoles starts from readily available ynamides. Subsequent reactions with various electrophiles provides polyfunctional N-heterocycles in good yields.
Glycosidation
Cooperative Catalysis in Glycosidation Reactions with O-Glycosyl Trichloroacetimidates as Glycosyl Donors†
- Pages: 10089-10092
- First Published: 26 July 2013
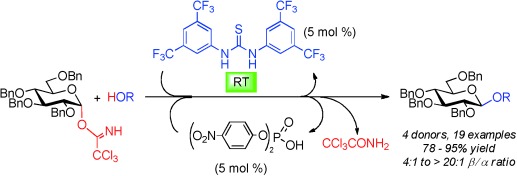
Thiourea mediates cooperative glycosidation through hydrogen bonding. N,N′-Diarylthiourea as cocatalyst enforces an SN2-type acid-catalyzed glycosidation even at room temperature (see scheme; Bn=benzyl). From O-(α-glycosyl) trichloroacetimidates as glycosyl donors and various acceptors, β-glycosides are preferentially or exclusively obtained.
Synthetic Methods
Full Functionalization of the 7-Azaindole Scaffold by Selective Metalation and Sulfoxide/Magnesium Exchange†
- Pages: 10093-10096
- First Published: 29 July 2013
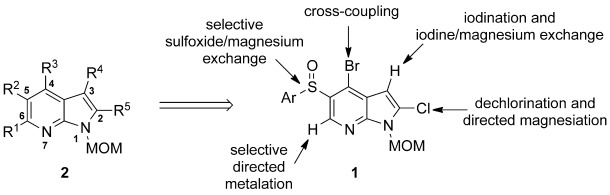
Filling positions: 7-Azaindoles are important targets in the pharmaceutical industry. All five carbon positions of the azaindole ring system can be functionalized in a predictable manner starting from the appropriately substituted azaindole 1 by directed metalation and halogen/magnesium and sulfoxide/magnesium exchange. The products are fully substituted azaindoles of type 2.
Self-Assembly
pH-Switchable Ampholytic Supramolecular Copolymers†
- Pages: 10097-10101
- First Published: 08 August 2013
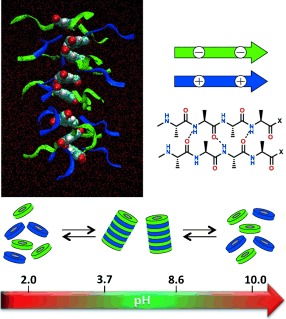
β-sheet-encoded anionic and cationic dendritic peptide amphiphiles form supramolecular copolymers when self-assembled in a 1:1 feed ratio of the monomers. These ampholytic materials have been designed for on-off polymerization in response to pH triggers. The cooperative supramolecular self-assembly process is switched on at a physiologically relevant pH value and can be switched off by increasing or decreasing the pH value.
Coordination Cages
Assembly and Stepwise Oxidation of Interpenetrated Coordination Cages Based on Phenothiazine†
- Pages: 10102-10106
- First Published: 23 July 2013
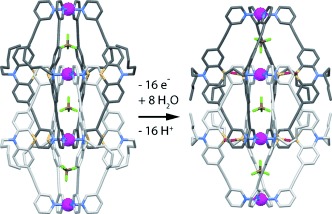
A breath of fresh air is sufficient for the eightfold S-monooxygenation of an interpenetrated double cage based on eight phenothiazine ligands and four square-planar-coordinated PdII cations. Besides these two cages, which were both characterized by X-ray crystallography, an eightfold S-dioxygenated double-cage was obtained under harsher oxidation conditions.
Hybrid Materials
Well-Defined Nanofibers with Tunable Morphology from Spherical Colloidal Building Blocks†
- Pages: 10107-10111
- First Published: 23 July 2013
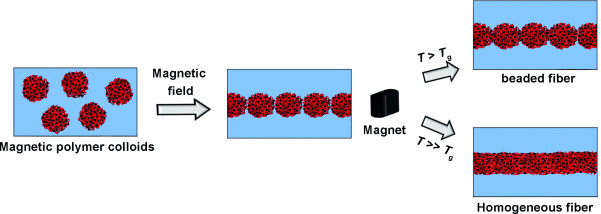
From particles to fibers: Nanofibers with different morphologies and periodicities can be fabricated by supraparticular assembly of magnetic spherical nanoparticles. A linear sintering process is used to merge the assembled colloids together. The structure of the obtained fibers is controlled by the process parameters and the morphology of the spherical colloidal building blocks.
Structural Biology
The Role of Water and Sodium Ions in the Activation of the μ-Opioid Receptor†
- Pages: 10112-10115
- First Published: 31 July 2013
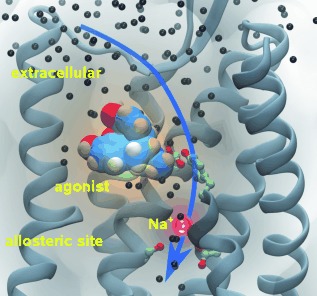
Dual effect of sodium ions: The activation of G-protein-coupled receptors depends on the presence of water molecules inside the receptor and also on allosteric interactions. The binding of sodium ions to the allosteric site of the μ opioid receptor was studied by microsecond molecular dynamics simulations and their seemingly contradictory roles in preventing ligand binding and facilitating receptor activation were explained.
Nitrogen Fixation
Analysis of the Magnetic Properties of Nitrogenase FeMo Cofactor by Single-Crystal EPR Spectroscopy†
- Pages: 10116-10119
- First Published: 08 August 2013
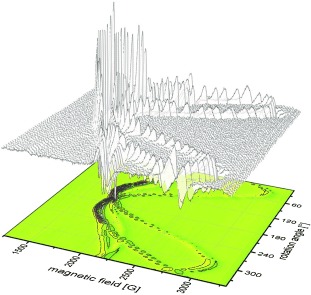
The catalytic center of nitrogenase, the [Mo:7Fe:9S:C]:homocitrate FeMo cofactor, is a S=3/2 system with a rhombic magnetic g tensor. Single-crystal EPR spectroscopy in combination with X-ray diffraction were used to determine the relative orientation of the g tensor with respect to the cluster structure. The protein environment influences the electronic structure of the FeMo cofactor, dictating preferred orientations of possible functional relevance.
Borylene Reduction
Reductive Borylene–CO Coupling with a Bulky Arylborylene Complex†
- Pages: 10120-10123
- First Published: 25 July 2013
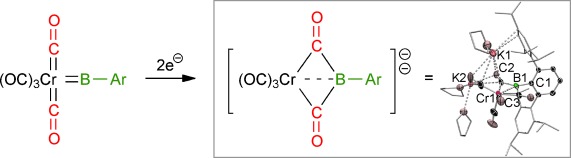
Partial metal–boron bond cleavage and coupling of a borylene with two CO ligands was observed upon reduction of a new bulky arylborylene complex. Both the borylene precursor and dianionic product were structurally and spectroscopically characterized. In contrast, reduction of an aminoborylene complex led to complete loss of the borylene ligand and classical Hieber reduction. A rationale for these differences based on DFT methods is presented.










![Simultaneous Self-Assembly of a [2]Catenane, a Trefoil Knot, and a Solomon Link from a Simple Pair of Ligands](/cms/asset/04bf4b45-5223-449d-a241-e63297c73640/mcontent.jpg)