Transition-Metal-Free Multicomponent Reactions Involving Arynes, N-Heterocycles, and Isatins†
Anup Bhunia
Organic Chemistry Division, CSIR-National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune (India) http://academic.ncl.res.in/ncl_1/at.biju
Search for more papers by this authorTony Roy
Organic Chemistry Division, CSIR-National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune (India) http://academic.ncl.res.in/ncl_1/at.biju
Search for more papers by this authorPradip Pachfule
Physical/Materials Chemistry Division, CSIR-National Chemical Laboratory (CSIR-NCL), Pune (India)
Search for more papers by this authorDr. Pattuparambil R. Rajamohanan
Central NMR Facility, CSIR-National Chemical Laboratory (CSIR-NCL), Pune (India)
Search for more papers by this authorCorresponding Author
Dr. Akkattu T. Biju
Organic Chemistry Division, CSIR-National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune (India) http://academic.ncl.res.in/ncl_1/at.biju
Organic Chemistry Division, CSIR-National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune (India) http://academic.ncl.res.in/ncl_1/at.biju===Search for more papers by this authorAnup Bhunia
Organic Chemistry Division, CSIR-National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune (India) http://academic.ncl.res.in/ncl_1/at.biju
Search for more papers by this authorTony Roy
Organic Chemistry Division, CSIR-National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune (India) http://academic.ncl.res.in/ncl_1/at.biju
Search for more papers by this authorPradip Pachfule
Physical/Materials Chemistry Division, CSIR-National Chemical Laboratory (CSIR-NCL), Pune (India)
Search for more papers by this authorDr. Pattuparambil R. Rajamohanan
Central NMR Facility, CSIR-National Chemical Laboratory (CSIR-NCL), Pune (India)
Search for more papers by this authorCorresponding Author
Dr. Akkattu T. Biju
Organic Chemistry Division, CSIR-National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune (India) http://academic.ncl.res.in/ncl_1/at.biju
Organic Chemistry Division, CSIR-National Chemical Laboratory (CSIR-NCL), Dr. Homi Bhabha Road, Pune (India) http://academic.ncl.res.in/ncl_1/at.biju===Search for more papers by this authorThis work was supported by CSIR-NCL (start-up grant to A.T.B., MLP022426), CSIR-New Delhi (Network project-ORIGIN, CSC0108) and CSIR-OSDD (HCP0001). A.B. and P.P. thank CSIR-New Delhi for the award of a Research Fellowship. We thank Dr. Rahul Banerjee for support with X-ray analysis, and B. Santhakumari for the HRMS data.
Graphical Abstract
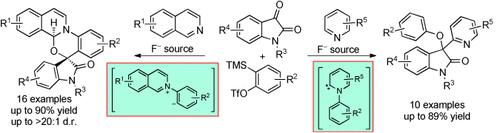
Mix and match: With isoquinoline as the nucleophilic trigger, multicomponent reactions afforded spirooxazino isoquinoline derivatives, proceeding through 1,4-dipolar intermediates. The use of pyridine as a nucleophile furnished indolin-2-one derivatives, with the reaction likely proceeding through a pyridylidene intermediate.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304278_sm_miscellaneous_information.pdf2.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on aryne chemistry, see:
- 1aP. M. Tadross, B. M. Stoltz, Chem. Rev. 2012, 112, 3550;
- 1bC. M. Gampe, E. M. Carreira, Angew. Chem. 2012, 124, 3829;
10.1002/ange.201107485 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3766;
- 1cA. Bhunia, S. R. Yetra, A. T. Biju, Chem. Soc. Rev. 2012, 41, 3140;
- 1dK. Okuma, Heterocycles 2012, 85, 515;
- 1e“Arylation reactions involving the formation of arynes”: Y. Chen, R. C. Larock in Modern Arylation Methods (Ed.: ), Wiley-VCH, Weinheim, 2009, p. 401;
10.1002/9783527627325.ch12 Google Scholar
- 1fH. Yoshida, J. Ohshita, A. Kunai, Bull. Chem. Soc. Jpn. 2010, 83, 199;
- 1gC. Wu, F. Shi, Asian J. Org. Chem. 2013, 2, 116;
- 1hA. V. Dubrovskiy, N. A. Markina, R. C. Larock, Org. Biomol. Chem. 2013, 11, 191;
- 1iR. Sanz, Org. Prep. Proced. Int. 2008, 40, 215.
- 2For selected recent reports, see:
- 2aD. A. Candito, D. Dobrovolsky, M. Lautens, J. Am. Chem. Soc. 2012, 134, 15572;
- 2bC. Lu, A. V. Dubrovskiy, R. C. Larock, J. Org. Chem. 2012, 77, 2279;
- 2cS. S. Bhojgude, T. Kaicharla, A. Bhunia, A. T. Biju, Org. Lett. 2012, 14, 4098;
- 2dT. Kaicharla, S. S. Bhojgude, A. T. Biju, Org. Lett. 2012, 14, 6238.
- 3For an account, see:
- 3aH. Yoshida, K. Takaki, Synlett 2012, 1725; for selected recent reports, see:
- 3bJ. Kim, B. M. Stoltz, Tetrahedron Lett. 2012, 53, 4994;
- 3cY. Fang, D. C. Rogness, R. C. Larock, F. Shi, J. Org. Chem. 2012, 77, 6262;
- 3dD. Rodríguez-Lojo, A. Cobas, D. Peña, D. Pérez, E. Guitián, Org. Lett. 2012, 14, 1363;
- 3eR. A. Dhokale, P. R. Thakare, S. B. Mhaske, Org. Lett. 2012, 14, 3994;
- 3fK. Mohanan, Y. Coquerel, J. Rodriguez, Org. Lett. 2012, 14, 4686.
- 4For selected recent reports, see:
- 4aC. Lu, A. V. Dubrovskiy, R. C. Larock, J. Org. Chem. 2012, 77, 8648;
- 4bK. Parthasarathy, H. Han, C. Prakash, C.-H. Cheng, Chem. Commun. 2012, 48, 6580.
- 5For selected recent reports, see:
- 5aK. M. Allan, C. D. Gilmore, B. M. Stoltz, Angew. Chem. 2011, 123, 4580;
10.1002/ange.201100911 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4488;
- 5bE. Yoshioka, S. Kohtani, H. Miyabe, Angew. Chem. 2011, 123, 6768;
10.1002/ange.201102088 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 6638;
- 5cH. Yoshida, Y. Ito, J. Ohshita, Chem. Commun. 2011, 47, 8512;
- 5dH. Yoshida, Y. Asatsu, Y. Mimura, Y. Ito, J. Ohshita, K. Takaki, Angew. Chem. 2011, 123, 9850; Angew. Chem. Int. Ed. 2011, 50, 9676.
- 6For a highlight, see: S. S. Bhojgude, A. T. Biju, Angew. Chem. 2012, 124, 1550;
10.1002/ange.201106984 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 1520.
- 7
- 7aH. Yoshida, H. Fukushima, J. Ohshita, A. Kunai, Angew. Chem. 2004, 116, 4025; Angew. Chem. Int. Ed. 2004, 43, 3935;
- 7bH. Yoshida, H. Fukushima, T. Morishita, J. Ohshita, A. Kunai, Tetrahedron 2007, 63, 4793.
- 8H. Yoshida, H. Fukushima, J. Ohshita, A. Kunai, J. Am. Chem. Soc. 2006, 128, 11040.
- 9
- 9aH. Yoshida, T. Morishita, J. Ohshita, Org. Lett. 2008, 10, 3845;
- 9bH. Yoshida, T. Morishita, H. Fukushima, J. Ohshita, A. Kunai, Org. Lett. 2007, 9, 3367;
- 9cT. Morishita, H. Fukushima, H. Yoshida, J. Ohshita, A. Kunai, J. Org. Chem. 2008, 73, 5452.
- 10
- 10aM. Jeganmohan, C.-H. Cheng, Chem. Commun. 2006, 2454;
- 10bM. Jeganmohan, S. Bhuvaneswari, C.-H. Cheng, Chem. Asian J. 2010, 5, 153.
- 11For recent reviews on isatins, see:
- 11aG. S. Singh, Z. Y. Desta, Chem. Rev. 2012, 112, 6104;
- 11bR. Dalpozzo, G. Bartolib, G. Bencivennib, Chem. Soc. Rev. 2012, 41, 7247.
- 12For selected reports on the generation of 1,4-dipolar intermediates from isoquinoline and activated CC bonds, see:
- 12aV. Nair, A. R. Sreekanth, N. Abhilash, M. M. Bhadbhade, R. C. Gonnade, Org. Lett. 2002, 4, 3575;
- 12bV. Nair, A. R. Sreekanth, A. T. Biju, N. P. Rath, Tetrahedron Lett. 2003, 44, 729.
- 13For selected reports, see:
- 13aJ. A. Cabeza, I. del Río, E. Pérez-Carreño, M. G. Sánchez-Vega, D. Vázquez-Garciá, Angew. Chem. 2009, 121, 563;
10.1002/ange.200804945 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 555;
- 13bM. Roselló-Merino, J. Díez, S. Conejero, Chem. Commun. 2010, 46, 9247;
- 13cK. Hata, Y. Segawa, K. Itami, Chem. Commun. 2012, 48, 6642; for a review, see: O. Schuster, L. Yang, H. G. Raubenheimer, M. Albrecht, Chem. Rev. 2009, 109, 3445.
- 14
- 14aY. Himeshima, T. Sonoda, H. Kobayashi, Chem. Lett. 1983, 1211;
- 14bD. Peña, A. Cobas, D. Pérez, E. Guitián, Synthesis 2002, 1454.
- 15For details, see the Supporting Information.
- 16CCDC-938921 (4 a), 938922 (8 a), 938923 (9 a), contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data request/cif.
- 17In this case, the pair of diastereomers arising from the addition of isoquinoline to the more hindered position of naphthalyne was also observed in ca. 5 % yield.
- 18For related reports on the generation of 1,4-dipolar intermediates from pyridine and activated CC bonds, see:
- 18aV. Nair, A. N. Pillai, R. S. Menon, E. Suresh, Org. Lett. 2005, 7, 1189;
- 18bV. Nair, A. R. Sreekanth, A. U. Vinod, Org. Lett. 2001, 3, 3495.
- 19For an earlier report on the addition of pyridine to aryne, see:
- 19aE. K. Fields, S. Meyerson, J. Org. Chem. 1966, 31, 3307; see also:
- 19bN. Dennis, A. R. Katritzky, S. K. Parton, J. Chem. Soc. Perkin Trans. 1 1976, 2285;
- 19cD. K. Rayabarapu, K. K. Majumdar, T. Sambaiah, C.-H. Cheng, J. Org. Chem. 2001, 66, 3646;
- 19dE. Ihara, A. Kurokawa, T. Koda, T. Muraki, T. Itoh, K. Inoue, Macromolecules 2005, 38, 2167.
- 20For the oxidation of nucleophilic heterocyclic carbenes in the presence of atmospheric oxygen, see:
- 20aH.-W. Wanzlick, E. Schikora, Chem. Ber. 1961, 94, 2389;
- 20bH.-W. Wanzlick, Angew. Chem. 1962, 74, 129; Angew. Chem. Int. Ed. 1962, 1, 75;
- 20cD. Enders, K. Breuer, J. Runsink, J. H. Teles, Liebigs Ann. 1996, 2019.
- 21Additionally, treating pyridine and aryne with elemental sulfur under degassing conditions resulted in the formation of 1-phenylpyridine-2(1H)-thione in 16 % yield. Although the yield is low, this is also an indication of the generation of a pyridylidene intermediate.
- 22It is reasonable to assume, in the case of electronically different aryne precursors 2 b and 2 c, that the electron-rich aryl ring (intermediate 14, Scheme 6) may hamper the SNAr reaction, hence the corresponding indolin-2-ones were not formed in these cases [Eq. (3)].
- 23The mechanistic difference in the reactivity of isoquinoline and pyridine towards aryne and isatin may be due to the less aromatic nature of the heterocyclic ring of isoquinoline, which facilitates the formation of spirooxazino derivatives. Owing to the relatively high aromaticity of pyridine, the corresponding spirocyclization is less favored in this case.