Molecular Mechanism of Prion Protein Oligomerization at Atomic Resolution†
Dr. Kai Schlepckow
Institut für Organische Chemie und Chemische Biologie, Zentrum für Biomolekulare Magnetische Resonanz (BMRZ), Johann Wolfgang Goethe-Universität Frankfurt am Main, Max-von-Laue-Strasse 7, 60438 Frankfurt am Main (Germany) http://schwalbe.org.chemie.uni-frankfurt.de
Search for more papers by this authorCorresponding Author
Prof. Dr. Harald Schwalbe
Institut für Organische Chemie und Chemische Biologie, Zentrum für Biomolekulare Magnetische Resonanz (BMRZ), Johann Wolfgang Goethe-Universität Frankfurt am Main, Max-von-Laue-Strasse 7, 60438 Frankfurt am Main (Germany) http://schwalbe.org.chemie.uni-frankfurt.de
Institut für Organische Chemie und Chemische Biologie, Zentrum für Biomolekulare Magnetische Resonanz (BMRZ), Johann Wolfgang Goethe-Universität Frankfurt am Main, Max-von-Laue-Strasse 7, 60438 Frankfurt am Main (Germany) http://schwalbe.org.chemie.uni-frankfurt.de===Search for more papers by this authorDr. Kai Schlepckow
Institut für Organische Chemie und Chemische Biologie, Zentrum für Biomolekulare Magnetische Resonanz (BMRZ), Johann Wolfgang Goethe-Universität Frankfurt am Main, Max-von-Laue-Strasse 7, 60438 Frankfurt am Main (Germany) http://schwalbe.org.chemie.uni-frankfurt.de
Search for more papers by this authorCorresponding Author
Prof. Dr. Harald Schwalbe
Institut für Organische Chemie und Chemische Biologie, Zentrum für Biomolekulare Magnetische Resonanz (BMRZ), Johann Wolfgang Goethe-Universität Frankfurt am Main, Max-von-Laue-Strasse 7, 60438 Frankfurt am Main (Germany) http://schwalbe.org.chemie.uni-frankfurt.de
Institut für Organische Chemie und Chemische Biologie, Zentrum für Biomolekulare Magnetische Resonanz (BMRZ), Johann Wolfgang Goethe-Universität Frankfurt am Main, Max-von-Laue-Strasse 7, 60438 Frankfurt am Main (Germany) http://schwalbe.org.chemie.uni-frankfurt.de===Search for more papers by this authorFinancial support of this work through the German Research Foundation (DFG, grant number Schw701/9-1,9-2) and through the state of Hessen (BMRZ) is gratefully acknowledged. H.S. is a member of the DFG-funded Cluster of Excellence: Macromolecular Complexes. We thank Nils Walter for helpful discussions, Julia Wirmer-Bartoschek for critically reading the manuscript, and Sven Warhaut for preparation of an NMR sample.
Graphical Abstract
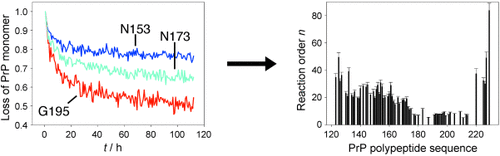
Prion protein oligomerization: Despite the crucial role of oligomers during prion protein (PrP) pathogenesis the molecular mechanism of their formation has remained largely elusive. A 2D time-resolved NMR study which made it possible to characterize the oligomerization kinetics with unprecedented site-specificity is reported (see picture).
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201305184_sm_miscellaneous_information.pdf1.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. B. Prusiner, Science 1982, 216, 136.
- 2S. B. Prusiner, Proc. Natl. Acad. Sci. USA 1998, 95, 13363.
- 3B. W. Caughey, A. Dong, K. S. Bhat, D. Ernst, S. F. Hayes, W. S. Caughey, Biochemistry 1991, 30, 7672.
- 4K. M. Pan, M. Baldwin, J. Nguyen, M. Gasset, A. Serban, D. Groth, I. Mehlhorn, Z. Huang, R. J. Fletterick, F. E. Cohen, et al., Proc. Natl. Acad. Sci. USA 1993, 90, 10962.
- 5R. Riek, S. Hornemann, G. Wider, M. Billeter, R. Glockshuber, K. Wüthrich, Nature 1996, 382, 180.
- 6D. A. Lysek, C. Schorn, L. G. Nivon, V. Esteve-Moya, B. Christen, L. Calzolai, C. von Schroetter, F. Fiorito, T. Herrmann, P. Güntert, K. Wüthrich, Proc. Natl. Acad. Sci. USA 2005, 102, 640.
- 7C. Gerum, R. Silvers, J. Wirmer-Bartoschek, H. Schwalbe, Angew. Chem. 2009, 121, 9616;
10.1002/ange.200903771 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9452.
- 8C. Gerum, K. Schlepckow, H. Schwalbe, J. Mol. Biol. 2010, 401, 7.
- 9X. Lu, P. L. Wintrode, W. K. Surewicz, Proc. Natl. Acad. Sci. USA 2007, 104, 1510.
- 10V. Smirnovas, G. S. Baron, D. K. Offerdahl, G. J. Raymond, B. Caughey, W. K. Surewicz, Nat. Struct. Mol. Biol. 2011, 18, 504.
- 11N. J. Cobb, F. D. Sonnichsen, H. McHaourab, W. K. Surewicz, Proc. Natl. Acad. Sci. USA 2007, 104, 18946.
- 12J. Kumar, S. Sreeramulu, T. L. Schmidt, C. Richter, J. Vonck, A. Heckel, C. Glaubitz, H. Schwalbe, ChemBioChem 2010, 11, 1208.
- 13R. Tycko, R. Savtchenko, V. G. Ostapchenko, N. Makarava, I. V. Baskakov, Biochemistry 2010, 49, 9488.
- 14C. Haass, D. J. Selkoe, Nat. Rev. Mol. Cell Biol. 2007, 8, 101.
- 15W. Swietnicki, M. Morillas, S. G. Chen, P. Gambetti, W. K. Surewicz, Biochemistry 2000, 39, 424.
- 16I. V. Baskakov, G. Legname, M. A. Baldwin, S. B. Prusiner, F. E. Cohen, J. Biol. Chem. 2002, 277, 21140.
- 17F. Sokolowski, A. J. Modler, R. Masuch, D. Zirwer, M. Baier, G. Lutsch, D. A. Moss, K. Gast, D. Naumann, J. Biol. Chem. 2003, 278, 40481.
- 18S. Jain, J. B. Udgaonkar, J. Mol. Biol. 2008, 382, 1228.
- 19M. Morillas, D. L. Vanik, W. K. Surewicz, Biochemistry 2001, 40, 6982.
- 20J. T. Jarrett, P. T. Lansbury, Jr., Cell 1993, 73, 1055.
- 21K. Kuwajima, Proteins 1989, 6, 87.
- 22C. Redfield, Methods 2004, 34, 121.
- 23R. Gerber, A. Tahiri-Alaoui, P. J. Hore, W. James, J. Biol. Chem. 2007, 282, 6300.
- 24J. M. Andrews, C. J. Roberts, J. Phys. Chem. B 2007, 111, 7897.
- 25A. J. Modler, H. Fabian, F. Sokolowski, G. Lutsch, K. Gast, G. Damaschun, Amyloid 2004, 11, 215.
- 26I. V. Baskakov, G. Legname, S. B. Prusiner, F. E. Cohen, J. Biol. Chem. 2001, 276, 19687.
- 27J. W. Donovan, Methods Enzymol. 1973, 27, 497.
- 28P. Neudecker, P. Robustelli, A. Cavalli, P. Walsh, P. Lundstrom, A. Zarrine-Afsar, S. Sharpe, M. Vendruscolo, L. E. Kay, Science 2012, 336, 362.
- 29A. J. Baldwin, S. J. Anthony-Cahill, T. P. Knowles, G. Lippens, J. Christodoulou, P. D. Barker, C. M. Dobson, Angew. Chem. 2008, 120, 3433;
10.1002/ange.200703915 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3385.
- 30N. Chakroun, S. Prigent, C. A. Dreiss, S. Noinville, C. Chapuis, F. Fraternali, H. Rezaei, FASEB J. 2010, 24, 3222.
- 31R. Gerber, K. Voitchovsky, C. Mitchel, A. Tahiri-Alaoui, J. F. Ryan, P. J. Hore, W. James, J. Mol. Biol. 2008, 381, 212.