A High-Resolution Structure that Provides Insight into Coiled-Coil Thiodepsipeptide Dynamic Chemistry†
Zehavit Dadon
Department of Chemistry, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Search for more papers by this authorDr. Manickasundaram Samiappan
Department of Chemistry, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Search for more papers by this authorDr. Anat Shahar
Institute of Biotechnology in the Negev, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Search for more papers by this authorDr. Raz Zarivach
Department of Life Sciences, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Institute of Biotechnology in the Negev, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Search for more papers by this authorCorresponding Author
Prof. Gonen Ashkenasy
Department of Chemistry, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Ilse Katz Institute for Nanoscale Science and Technology, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Department of Chemistry, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)===Search for more papers by this authorZehavit Dadon
Department of Chemistry, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Search for more papers by this authorDr. Manickasundaram Samiappan
Department of Chemistry, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Search for more papers by this authorDr. Anat Shahar
Institute of Biotechnology in the Negev, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Search for more papers by this authorDr. Raz Zarivach
Department of Life Sciences, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Institute of Biotechnology in the Negev, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Search for more papers by this authorCorresponding Author
Prof. Gonen Ashkenasy
Department of Chemistry, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Ilse Katz Institute for Nanoscale Science and Technology, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)
Department of Chemistry, Ben Gurion University of the Negev, Beer Sheva, 84105 (Israel)===Search for more papers by this authorThis research was supported by the European Research Council (ERC 259204). We thank Vered Zavaro for assistance in early stages of the project, and Dr. Rivka Cohen-Luria for help in the lab.
Graphical Abstract
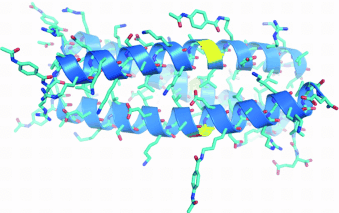
Stable and reactive: A crystal structure at 1.35 Å of a thioester coiled-coil protein reveals high similarity to all-peptide-bond proteins. In these assemblies, the thioester bonds are kept reactive towards thiol molecules in the mixture. This enables efficient domain exchange between proteins in response to changes in folding conditions or introduction of external templates.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303900_sm_miscellaneous_information.pdf3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1C. C. Liu, P. G. Schultz, Annu. Rev. Biochem. 2010, 79, 413–444.
- 2Z. Hao, S. Hong, X. Chen, P. R. Chen, Acc. Chem. Res. 2011, 44, 742–751.
- 3S. H. Gellman, Acc. Chem. Res. 1998, 31, 173–180.
- 4W. S. Horne, S. H. Gellman, Acc. Chem. Res. 2008, 41, 1399–1408.
- 5W. S. Horne, Expert Opin. Drug Discovery 2011, 6, 1247–1262.
- 6E. T. Powers, S. Deechongkit, J. W. Kelly, Adv. Protein Chem. 2006, 59, 39–78.
- 7J. A. Scheike, C. Baldauf, J. Spengler, F. Albericio, M. T. Pisabarro, B. Koksch, Angew. Chem. 2007, 119, 7912–7916;
10.1002/ange.200702218 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7766–7769.
- 8J. Gao, D. A. Bosco, E. T. Powers, J. W. Kelly, Nat. Struct. Mol. Biol. 2009, 16, 684–690.
- 9A. Choudhary, R. T. Raines, ChemBioChem 2011, 12, 1801–1807.
- 10M. G. Woll, S. H. Gellman, J. Am. Chem. Soc. 2004, 126, 11172–11174.
- 11A. Koglin, C. T. Walsh, Nat. Prod. Rep. 2009, 26, 987–1000.
- 12Y. Ura, J. M. Beierle, L. J. Leman, L. E. Orgel, M. R. Ghadiri, Science 2009, 325, 73–77.
- 13Y. Ura, M. Al-Sayah, J. Montenegro, J. M. Beierle, L. J. Leman, M. R. Ghadiri, Org. Biomol. Chem. 2009, 7, 2878–2884.
- 14Z. Dadon, M. Samiappan, N. Wagner, G. Ashkenasy, Chem. Commun. 2012, 48, 1419–1421.
- 15S. Ghosh, L. A. Ingerman, A. G. Frye, S. J. Lee, M. R. Gagne, M. L. Waters, Org. Lett. 2010, 12, 1860–1863.
- 16R. F. Ludlow, S. Otto, Chem. Soc. Rev. 2008, 37, 101–108.
- 17Z. Dadon, N. Wagner, G. Ashkenasy, Angew. Chem. 2008, 120, 6221–6230;
10.1002/ange.200702552 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6128–6136.
- 18S. Yao, I. Ghosh, R. Zutshi, J. Chmielewski, Nature 1998, 396, 447–450.
- 19A. Saghatelian, Y. Yokobayashi, K. Soltani, M. R. Ghadiri, Nature 2001, 409, 797–801.
- 20G. Ashkenasy, R. Jagasia, M. Yadav, M. R. Ghadiri, Proc. Natl. Acad. Sci. USA 2004, 101, 10872–10877.
- 21Z. Dadon, M. Samiappan, E. Y. Safranchik, G. Ashkenasy, Chem. Eur. J. 2010, 16, 12096–12099.
- 22P. B. Harbury, T. Zhang, P. S. Kim, T. Alber, Science 1993, 262, 1401–1407.
- 23J. L. Price, E. B. Hadley, J. D. Steinkruger, S. H. Gellman, Angew. Chem. 2010, 122, 378–381;
10.1002/ange.200904714 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 368–371.
- 24E. B. Hadley, O. D. Testa, D. N. Woolfson, S. H. Gellman, Proc. Natl. Acad. Sci. USA 2008, 105, 530–535.
- 25J. D. Steinkruger, D. N. Woolfson, S. H. Gellman, J. Am. Chem. Soc. 2010, 132, 7586–7588.
- 26J. D. Steinkruger, G. J. Bartlett, D. N. Woolfson, S. H. Gellman, J. Am. Chem. Soc. 2012, 134, 15652–15655.
- 27P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders, S. Otto, Chem. Rev. 2006, 106, 3652–3711.
- 28R. L. E. Furlan, Y.-F. Ng, S. Otto, J. K. M. Sanders, J. Am. Chem. Soc. 2001, 123, 8876–8877.
- 29M. Rauschenberg, S. Bomke, U. Karst, B. J. Ravoo, Angew. Chem. 2010, 122, 7498–7503;
10.1002/ange.201002847 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7340–7345.
- 30J. M. A. Carnall, C. A. Waudby, A. M. Belenguer, M. C. A. Stuart, J. J. P. Peyralans, S. Otto, Science 2010, 327, 1502–1506.
- 31J. Atcher, A. Moure, I. Alfonso, Chem. Commun. 2013, 49, 487–489.