Assembly and Stepwise Oxidation of Interpenetrated Coordination Cages Based on Phenothiazine†
Marina Frank
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de
Search for more papers by this authorJakob Hey
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de
Search for more papers by this authorIlker Balcioglu
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de
Search for more papers by this authorDr. Yu-Sheng Chen
Center for Advanced Radiation Source (ChemMatCARS), The University of Chicago c/o APS/ANL (USA)
Search for more papers by this authorProf. Dr. Dietmar Stalke
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de
Search for more papers by this authorProf. Dr. Tomoyoshi Suenobu
Department of Material and Life Science, Graduate School of Engineering, Osaka University, ALCA, Japan Science and Technology Agency (JST), Suita, Osaka 565-0871 (Japan)
Search for more papers by this authorProf. Dr. Shunichi Fukuzumi
Department of Material and Life Science, Graduate School of Engineering, Osaka University, ALCA, Japan Science and Technology Agency (JST), Suita, Osaka 565-0871 (Japan)
Department of Bioinspired Science, Ewha Womans University, Seoul 120-750 (Korea)
Search for more papers by this authorDr. Holm Frauendorf
Institute for Organic and Biomolecular Chemistry, Georg-August University Göttingen, Tammannstrasse 2, 37077 Göttingen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Guido H. Clever
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de===Search for more papers by this authorMarina Frank
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de
Search for more papers by this authorJakob Hey
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de
Search for more papers by this authorIlker Balcioglu
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de
Search for more papers by this authorDr. Yu-Sheng Chen
Center for Advanced Radiation Source (ChemMatCARS), The University of Chicago c/o APS/ANL (USA)
Search for more papers by this authorProf. Dr. Dietmar Stalke
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de
Search for more papers by this authorProf. Dr. Tomoyoshi Suenobu
Department of Material and Life Science, Graduate School of Engineering, Osaka University, ALCA, Japan Science and Technology Agency (JST), Suita, Osaka 565-0871 (Japan)
Search for more papers by this authorProf. Dr. Shunichi Fukuzumi
Department of Material and Life Science, Graduate School of Engineering, Osaka University, ALCA, Japan Science and Technology Agency (JST), Suita, Osaka 565-0871 (Japan)
Department of Bioinspired Science, Ewha Womans University, Seoul 120-750 (Korea)
Search for more papers by this authorDr. Holm Frauendorf
Institute for Organic and Biomolecular Chemistry, Georg-August University Göttingen, Tammannstrasse 2, 37077 Göttingen (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Guido H. Clever
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de
Institute for Inorganic Chemistry, Georg-August University Göttingen, Tammannstrasse 4, 37077 Göttingen (Germany) http://www.clever-lab.de===Search for more papers by this authorM.F. thanks the Evonik Foundation and J.H. the CaSuS program of Lower Saxony for PhD fellowships. We thank the DFG (CL 489/2-1), the FCI, and the HeKKSaGOn consortium for support, and Dr. M. John for NMR measurements. ChemMatCARS and the Advanced Photon Source are supported by the NSF Department of Energy (NSF/CHE-0822838, DE-AC02-06CH11357).
Graphical Abstract
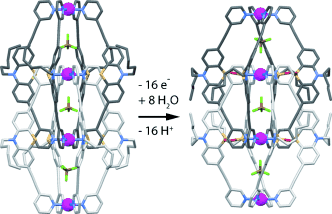
A breath of fresh air is sufficient for the eightfold S-monooxygenation of an interpenetrated double cage based on eight phenothiazine ligands and four square-planar-coordinated PdII cations. Besides these two cages, which were both characterized by X-ray crystallography, an eightfold S-dioxygenated double-cage was obtained under harsher oxidation conditions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302536_sm_miscellaneous_information.pdf2.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. Fujita, K. Umemoto, M. Yoshizawa, N. Fujita, T. Kusukawa, K. Biradha, Chem. Commun. 2001, 509;
- 1bS. J. Dalgarno, N. P. Power, J. L. Atwood, Coord. Chem. Rev. 2008, 252, 825;
- 1cD. Tranchemontagne, Z. Ni, M. O’Keeffe, O. Yaghi, Angew. Chem. 2008, 120, 5214;
10.1002/ange.200705008 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5136;
- 1dR. Chakrabarty, P. S. Mukherjee, P. J. Stang, Chem. Rev. 2011, 111, 6810;
- 1eT. K. Ronson, S. Zarra, S. P. Black, J. R. Nitschke, Chem. Commun. 2013, 49, 2476.
- 2G. H. Clever in Molecules at Work (Ed.: ), Wiley-VCH, Weinheim, 2012.
10.1002/9783527645787.ch2 Google Scholar
- 3
- 3aJ. L. Sessler, P. Gale, W.-S. Cho, S. J. Rowan, Anion Receptor Chemistry (Monographs in Supramolecular Chemistry), Royal Society of Chemistry, 2006;
10.1039/9781847552471 Google Scholar
- 3bR. Custelcean, J. Bosano, P. V. Bonnesen, V. Kertesz, B. P. Hay, Angew. Chem. 2009, 121, 4085;
10.1002/ange.200900108 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 4025;
- 3cS. O. Kang, J. M. Llinares, V. W. Day, K. Bowman-James, Chem. Soc. Rev. 2010, 39, 3980;
- 3dM. Wang, V. Vajpayee, S. Shanmugaraju, Y.-R. Zheng, Z. Zhao, H. Kim, P. S. Mukherjee, K.-W. Chi, P. J. Stang, Inorg. Chem. 2011, 50, 1506;
- 3eJ. A. Thomas, Dalton Trans. 2011, 40, 12005;
- 3fG. H. Clever, W. Kawamura, M. Shionoya, Inorg. Chem. 2011, 50, 4689;
- 3gG. H. Clever, W. Kawamura, S. Tashiro, M. Shiro, M. Shionoya, Angew. Chem. 2012, 124, 2660;
10.1002/ange.201108197 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 2606.
- 4P. Mal, B. Breiner, K. Rissanen, J. R. Nitschke, Science 2009, 324, 1697.
- 5
- 5aP. Mal, D. Schultz, K. Beyeh, K. Rissanen, J. R. Nitschke, Angew. Chem. 2008, 120, 8421;
10.1002/ange.200803066 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 8297;
- 5bF. Schmitt, J. Freudenreich, N. P. E. Barry, L. Juillerat-Jeanneret, G. Süss-Fink, B. Therrien, J. Am. Chem. Soc. 2012, 134, 754;
- 5cZ. Ma, B. Moulton, Coord. Chem. Rev. 2011, 255, 1623;
- 5dJ. E. M. Lewis, E. L. Gavey, S. A. Cameron, J. D. Crowley, Chem. Sci. 2012, 3, 778.
- 6
- 6aM. Ziegler, J. Brumaghim, K. Raymond, Angew. Chem. 2000, 112, 4285;
Angew. Chem. Int. Ed. 2000, 39, 4119;
10.1002/1521-3773(20001117)39:22<4119::AID-ANIE4119>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 6bD. Fiedler, R. G. Bergman, K. N. Raymond, Angew. Chem. 2006, 118, 759; Angew. Chem. Int. Ed. 2006, 45, 745;
- 6cM. Kawano, Y. Kobayashi, T. Ozeki, M. Fujita, J. Am. Chem. Soc. 2006, 128, 6558;
- 6dC.-Y. Gao, L. Zhao, M.-X. Wang, J. Am. Chem. Soc. 2012, 134, 824.
- 7
- 7aD. Vriezema, M. Aragones, J. Elemans, J. Cornelissen, A. Rowan, R. Nolte, Chem. Rev. 2005, 105, 1445;
- 7bM. Yoshizawa, J. K. Klosterman, M. Fujita, Angew. Chem. 2009, 121, 3470; Angew. Chem. Int. Ed. 2009, 48, 3418;
- 7c Molecular Encapsulation: Organic Reactions in Constrained Systems (Eds.: ), Wiley, Hoboken, 2010;
- 7dM. J. Wiester, P. A. Ulmann, C. A. Mirkin, Angew. Chem. 2011, 123, 118; Angew. Chem. Int. Ed. 2011, 50, 114.
- 8
- 8aJ. K. Klosterman, M. Iwamura, T. Tahara, M. Fujita, J. Am. Chem. Soc. 2009, 131, 9478;
- 8bM. Han, R. Michel, B. He, Y.-S. Chen, D. Stalke, M. John, G. H. Clever, Angew. Chem. 2013, 125, 1358; Angew. Chem. Int. Ed. 2013, 52, 1319.
- 9
- 9aT. Hasobe, Phys. Chem. Chem. Phys. 2010, 12, 44;
- 9bP. A. Troshin, N. S. Sariciftci in Supramolecular Chemistry—From Molecules to Nanomaterials (Eds.: ), Wiley, Chichester, UK, 2012.
- 10R. L. Carroll, C. B. Gorman, Angew. Chem. 2002, 114, 4556;
10.1002/1521-3757(20021202)114:23<4556::AID-ANGE4556>3.0.CO;2-W Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4378.10.1002/1521-3773(20021202)41:23<4378::AID-ANIE4378>3.0.CO;2-A CAS PubMed Web of Science® Google Scholar
- 11S. Bivaud, J.-Y. Balandier, M. Chas, M. Allain, S. Goeb, M. Sallé, J. Am. Chem. Soc. 2012, 134, 11968.
- 12G. A. Ozin, A. C. Arsenault, L. Cademartiri, Nanochemistry: a Chemical Approach to Nanomaterials, RSC, London, 2009.
- 13M. J. Ohlow, B. Moosmann, Drug Discovery Today 2011, 16, 119.
- 14T. Manju, N. Manoj, A. M. Braun, E. Oliveros, Photochem. Photobiol. Sci. 2012, 11, 1744.
- 15E. E. Bancroft, J. E. Pemberton, H. N. Blount, J. Phys. Chem. 1980, 84, 2557.
- 16
- 16aD. G. McCafferty, D. A. Friesen, E. Danielson, C. G. Wall, M. J. Saderholm, B. W. Erickson, T. J. Meyer, Proc. Natl. Acad. Sci. USA 1996, 93, 8200;
- 16bM. Borgström, O. Johansson, R. Lomoth, H. B. Baudin, S. Wallin, L. Sun, B. Akermark, L. Hammarström, Inorg. Chem. 2003, 42, 5173;
- 16cE. A. Weiss, M. J. Ahrens, L. E. Sinks, A. V. Gusev, M. A. Ratner, M. R. Wasielewski, J. Am. Chem. Soc. 2004, 126, 5577;
- 16dH. Tian, X. Yang, R. Chen, Y. Pan, L. Li, A. Hagfeldt, L. Sun, Chem. Commun. 2007, 3741;
- 16eK. Kawai, Y. Osakada, M. Fujitsuka, T. Majima, J. Phys. Chem. B 2008, 112, 2144;
- 16fM. Marszalek, S. Nagane, A. Ichake, R. Humphry-Baker, V. Paul, S. M. Zakeeruddin, M. Grätzel, J. Mater. Chem. 2012, 22, 889;
- 16gP. K. Poddutoori, A. S. D. Sandanayaka, N. Zarrabi, T. Hasobe, O. Ito, A. van der Est, J. Phys. Chem. A 2011, 115, 709.
- 17V. Balzani, M. Clemente-Leon, A. Credi, B. Ferrer, M. Venturi, A. H. Flood, J. F. Stoddart, Proc. Natl. Acad. Sci. USA 2006, 103, 1178.
- 18
- 18aC. Buhrmester, L. Moshurchak, R. L. Wang, J. R. Dahn, J. Electrochem. Soc. 2006, 153, A 288;
- 18b Lithium-Ion Batteries: Advanced Materials and Technologies (Eds.: ), CRC, Boca Raton, 2011.
- 19
- 19aC. S. Krämer, T. J. J. Müller, Eur. J. Org. Chem. 2003, 3534;
- 19bM. Hauck, J. Schönhaber, A. J. Zucchero, K. I. Hardcastle, T. J. J. Müller, U. H. F. Bunz, J. Org. Chem. 2007, 72, 6714;
- 19cM. Sailer, A. W. Franz, T. J. J. Müller, Chem. Eur. J. 2008, 14, 2602.
- 20K. Memminger, T. Oeser, T. J. J. Müller, Org. Lett. 2008, 10, 2797.
- 21For another example of oligophenothiazines, see T. Okamoto, M. Kuratsu, M. Kozaki, K. Hirotsu, A. Ichimura, T. Matsushita, K. Okada, Org. Lett. 2004, 6, 3493; for another example of a metal-mediated ring compound based on phenothiazine, see D. Li, X. Tian, G. Hu, Q. Zhang, P. Wang, P. Sun, H. Zhou, X. Meng, J. Yang, J. Wu, B. Jin, S. Zhang, X. Tao, Y. Tian, Inorg. Chem. 2011, 50, 7997.
- 22
- 22aJ. E. Beves, B. A. Blight, C. J. Campbell, D. A. Leigh, R. T. McBurney, Angew. Chem. 2011, 123, 9428;
10.1002/ange.201007963 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 9260;
- 22bR. S. Forgan, J.-P. Sauvage, J. F. Stoddart, Chem. Rev. 2011, 111, 5434;
- 22cJ.-F. Ayme, J. E. Beves, C. J. Campbell, D. A. Leigh, Chem. Soc. Rev. 2013, 42, 1700;
- 22dD. M. Engelhard, S. Freye, K. Grohe, M. John, G. H. Clever, Angew. Chem. 2012, 124, 4828;
10.1002/ange.201200611 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 4747.
- 23
- 23aJ. W. Steed, J. L. Atwood, Supramolecular Chemistry, Wiley, Hoboken, 2009;
10.1002/9780470740880 Google Scholar
- 23bH.-J. Schneider, Angew. Chem. 2009, 121, 3982;
10.1002/ange.200802947 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3924.
- 24
- 24aS. Freye, J. Hey, A. Torras Galán, D. Stalke, R. Herbst-Irmer, M. John, G. H. Clever, Angew. Chem. 2012, 124, 2233;
10.1002/ange.201107184 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 2191;
- 24bJ. M. Dieterich, G. H. Clever, R. A. Mata, Phys. Chem. Chem. Phys. 2012, 14, 12746.
- 25S. Freye, R. Michel, D. Stalke, M. Pawliczek, H. Frauendorf, G. H. Clever, J. Am. Chem. Soc. 2013, 135, 8476.
- 26
- 26aM. Fukuda, R. Sekiya, R. Kuroda, Angew. Chem. 2008, 120, 718;
10.1002/ange.200703162 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 706;
- 26bR. Sekiya, M. Fukuda, R. Kuroda, J. Am. Chem. Soc. 2012, 134, 10987.
- 27L. Găină, A. Csámpai, G. Túrós, T. Lovász, V. Zsoldos-Mády, I. A. Silberg, P. Sohár, Org. Biomol. Chem. 2006, 4, 4375.
- 28Q. Wang, L. Yang, Z. Xu, Y. Sun, Acta Crystallogr. Sect. E 2009, 65, o 1978.
- 29Gas-phase DTF calculations (B3LYP/6-311++G(d,p)) of 10-methyl-10H-phenothiazine-5-oxide yield a 2 kJ mol−1 energetic difference between the pseudo-axial and -equatorial isomers, and a 22 kJ mol−1 ring-flip barrier.
- 30T. Ishihara, H. Kakuta, H. Moritani, T. Ugawa, I. Yanagisawa, Chem. Pharm. Bull. 2004, 52, 1204.