Full Functionalization of the 7-Azaindole Scaffold by Selective Metalation and Sulfoxide/Magnesium Exchange†
M. Sc. Nadja M. Barl
Department Chemie, Ludwig-Maximilians-Universität München, Butenandtstrasse 5–13, 81377 München (Germany)
Search for more papers by this authorDr. Elodie Sansiaume-Dagousset
Department Chemie, Ludwig-Maximilians-Universität München, Butenandtstrasse 5–13, 81377 München (Germany)
Search for more papers by this authorProf. Dr. Konstantin Karaghiosoff
Department Chemie, Ludwig-Maximilians-Universität München, Butenandtstrasse 5–13, 81377 München (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Paul Knochel
Department Chemie, Ludwig-Maximilians-Universität München, Butenandtstrasse 5–13, 81377 München (Germany)
Department Chemie, Ludwig-Maximilians-Universität München, Butenandtstrasse 5–13, 81377 München (Germany)===Search for more papers by this authorM. Sc. Nadja M. Barl
Department Chemie, Ludwig-Maximilians-Universität München, Butenandtstrasse 5–13, 81377 München (Germany)
Search for more papers by this authorDr. Elodie Sansiaume-Dagousset
Department Chemie, Ludwig-Maximilians-Universität München, Butenandtstrasse 5–13, 81377 München (Germany)
Search for more papers by this authorProf. Dr. Konstantin Karaghiosoff
Department Chemie, Ludwig-Maximilians-Universität München, Butenandtstrasse 5–13, 81377 München (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Paul Knochel
Department Chemie, Ludwig-Maximilians-Universität München, Butenandtstrasse 5–13, 81377 München (Germany)
Department Chemie, Ludwig-Maximilians-Universität München, Butenandtstrasse 5–13, 81377 München (Germany)===Search for more papers by this authorWe thank the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013; ERC grant agreement no. 227763) and Novartis (Basel) for financial support. We also thank BASF AG (Ludwigshafen), W. C. Heraeus (Hanau), and Chemetall GmbH (Frankfurt) for generous gifts of chemicals.
Graphical Abstract
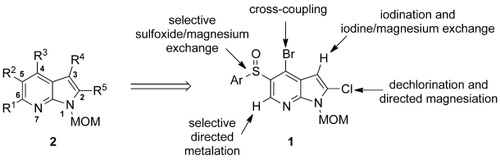
Filling positions: 7-Azaindoles are important targets in the pharmaceutical industry. All five carbon positions of the azaindole ring system can be functionalized in a predictable manner starting from the appropriately substituted azaindole 1 by directed metalation and halogen/magnesium and sulfoxide/magnesium exchange. The products are fully substituted azaindoles of type 2.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303490_sm_miscellaneous_information.pdf2.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews on the synthesis and properties of azaindole derivatives, see:
- 1aJ.-Y. Mérour, B. Joseph, Curr. Org. Chem. 2001, 5, 471;
- 1bF. Popowycz, S. Routier, B. Joseph, J.-Y. Mérour, Tetrahedron 2007, 63, 1031;
- 1cF. Popowycz, J.-Y. Mérour, B. Joseph, Tetrahedron 2007, 63, 8689;
- 1dJ. Alvarez-Builla, J. J. Vaquero, J. Barluenga in Modern Heterocyclic Chemistry, Vol. 4 (Eds.: ), Wiley-VCH, Weinheim, 2011;
- 1eC. Lamberth, J. Dinges in Bioactive Heterocyclic Compound Classes, Vol. 1 (Eds.: ), Wiley-VCH, Weinheim, 2012;
10.1002/9783527664412 Google Scholar
- 1fZ. Wang, X. Wang, Prog. Chem. 2012, 24, 1974;
- 1gJ.-Y. Mérour, S. Routier, F. Suzenet, B. Joseph, Tetrahedron 2013, 69, 4767;
- 1hY. Q. Fang, J. Yuen, M. Lautens, J. Org. Chem. 2007, 72, 5152. For studies on cytotoxical activity of azaindoles, see:
- 1iA. Echalier, K. Bettayeb, Y. Ferandin, O. Lozach, M. Clément, A. Valette, F. Liger, B. Marquet, J. C. Morris, J. A. Endicott, B. Joseph, L. Meijer, J. Med. Chem. 2008, 51, 737;
- 1jD. P. Power, O. Lozach, L. Meijer, D. H. Grayson, S. Connon, Bioorg. Med. Chem. Lett. 2010, 20, 4940.
- 2
- 2aQ. Wu, M. Estaghamatian, N.-X. Hu, Z. Popovic, G. Enright, S. R. Breeze, S. Wang, Angew. Chem. 1999, 111, 1039;
Angew. Chem. Int. Ed. 1999, 38, 985;
10.1002/(SICI)1521-3773(19990401)38:7<985::AID-ANIE985>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 2bQ. Wu, A. Hook, S. Wang, Angew. Chem. 2000, 112, 4094; Angew. Chem. Int. Ed. 2000, 39, 3933.
- 3C. Waloch, J. Wieland, M. Keller, B. Breit, Angew. Chem. 2007, 119, 3097;
10.1002/ange.200605202 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3037.
- 4
- 4aE. Fischer, F. Jourdan, Ber. Dtsch. Chem. Ges. 1883, 16, 2241;
10.1002/cber.188301602141 Google Scholar
- 4bM. Inman, C. J. Moody, Chem. Sci. 2013, 4, 29;
- 4cApplication of the Fischer indole synthesis for the preparation of 7-azaindoles: H. Kroth (AC Immune S.A.), WO 2011/128455, 2011.
- 5M. Nazaré, C. Schneider, A. Lindenschmidt, D. W. Will, Angew. Chem. 2004, 116, 4626;
10.1002/ange.200460122 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 4526.
- 6
- 6aR. C. Larock, E. K. Yum, M. D. Refvik, J. Org. Chem. 1998, 63, 7652;
- 6bG. R. Humphrey, J. T. Kuethe, Chem. Rev. 2006, 106, 2875;
- 6cApplication of the Larock indole synthesis for the preparation of 7-azaindoles: S. Park, J.-K. Choi, E. K. Yum, D.-C. Ha, Tetrahedron Lett. 1998, 39, 627.
- 7J. L. Henderson, S. M. McDermott, S. L. Buchwald, Org. Lett. 2010, 12, 4438.
- 8C. Schneider, E. David, A. A. Toutov, V. Snieckus, Angew. Chem. 2012, 124, 2776;
10.1002/ange.201108016 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 2722.
- 9
- 9aJ. Chen, Q. Song, C. Wang, Z. Xi, J. Am. Chem. Soc. 2002, 124, 6238;
- 9bK. Snegaroff, S. Komagawa, F. Chevallier, P. C. Gros, S. Golhen, T. Roisnel, M. Uchiyama, F. Mongin, Chem. Eur. J. 2010, 16, 8191;
- 9cR. E. Mulvey, F. Mongin, M. Uchiyama, Y. Kondo, Angew. Chem. 2007, 119, 3876; Angew. Chem. Int. Ed. 2007, 46, 3802;
- 9dF. Chevallier, F. Mongin, Chem. Soc. Rev. 2008, 37, 595;
- 9eA. Seggio, F. Chevallier, M. Vaultier, F. Mongin, J. Org. Chem. 2007, 72, 6602.
- 10
- 10aP. Knochel, W. Dohle, N. Gommermann, F. F. Kneisel, F. Kopp, T. Korn, I. Sapountzis, V. A. Vu, Angew. Chem. 2003, 115, 4438;
10.1002/ange.200300579 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4302;
- 10bI. Sapountzis, P. Knochel, Angew. Chem. 2002, 114, 1680;
10.1002/1521-3757(20020503)114:9<1680::AID-ANGE1680>3.0.CO;2-# Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1610;10.1002/1521-3773(20020503)41:9<1610::AID-ANIE1610>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 10cA. Krasovskiy, P. Knochel, Angew. Chem. 2004, 116, 3396;
10.1002/ange.200454084 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3333;
- 10dH. Ren, P. Knochel, Chem. Commun. 2006, 726;
- 10eC.-Y. Liu, P. Knochel, Org. Lett. 2005, 7, 2543;
- 10fN. Boudet, P. Knochel, Org. Lett. 2006, 8, 3737;
- 10gF. Kopp, A. Krasovskiy, P. Knochel, Chem. Commun. 2004, 2288.
- 11
- 11aT. Satoh, D. Taguchi, C. Suzuki, S. Fujisawa, Tetrahedron 2001, 57, 493;
- 11bT. Satoh, K. Takano, H. Someya, K. Matsuda, Tetrahedron Lett. 1995, 36, 7097;
- 11cT. Satoh, K. Takano, H. Ota, H. Someya, K. Matsuda, K. Yamakawa, Tetrahedron 1998, 54, 5557;
- 11dFor a review, see: T. Satoh, Chem. Soc. Rev. 2007, 36, 1561;
- 11eT. Satoh, K. Akita, Chem. Pharm. Bull. 2003, 51, 181;
- 11fT. Satoh, M. Miura, K. Sakai, Y. Yokoyama, Tetrahedron 2006, 62, 4253;
- 11gS. Sugiyama, H. Shimizu, T. Satoh, Tetrahedron Lett. 2006, 47, 8771.
- 12
- 12aL. Melzig, C. B. Rauhut, P. Knochel, Chem. Commun. 2009, 3536;
- 12bL. Melzig, C. B. Rauhut, N. Naredi-Rainer, P. Knochel, Chem. Eur. J. 2011, 17, 5362.
- 13S. Hildbrand, H.-J. Mair, R.-N. Radinov, Y. Ren, J. Anderson Wright, US 2011/0028511, 2011.
- 14K. Sonogashira, Y. Thoda, N. Hagihara, Tetrahedron Lett. 1975, 16, 4467–4470.
- 15F. Monnier, F. Turtaut, L. Duroure, M. Taillefer, Org. Lett. 2008, 10, 3203.
- 16C. He, J. Ke, H. Xu, A. Lei, Angew. Chem. 2013, 125, 1567; Angew. Chem. Int. Ed. 2013, 52, 1527.
- 17J. J. Cui (Pfizer Company), WO 2009/016460, 2009.
- 18C. Koradin, W. Dohle, A. L. Rodriguez, B. Schmid, P. Knochel, Tetrahedron 2003, 59, 1571.
- 19F. Chemla, I. Marek, J.-F. Normant, Synlett 1993, 665.
- 20For the preparation of 4-methoxybenzenesulfinyl chloride, see: M. Peyronneau, N. Roques, S. Mazieres, C. Le Roux, Synlett 2003, 631.
- 21
- 21aA. Krasovskiy, V. Krasovskaya, P. Knochel, Angew. Chem. 2006, 118, 3024;
10.1002/ange.200504024 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2958;
- 21bB. Haag, M. Mosrin, H. Ila, V. Malakhov, P. Knochel, Angew. Chem. 2011, 123, 9968;
10.1002/ange.201101960 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 9794.
- 22The regiochemistry of the products 1, 10, 11, 14 a, 14 b, and 22 was confirmed by X-ray crystallography (see the Supporting Information). CCDC 935382 (1), 935383 (10), 935384 (11), 935385 (14 a), 935386 (14 b), and 935387 (22) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 23
- 23aP. Knochel, M. Yeh, S. Berk, J. Talbert, J. Org. Chem. 1988, 53, 2390;
- 23bF. Dübner, P. Knochel, Angew. Chem. 1999, 111, 391;
10.1002/(SICI)1521-3757(19990201)111:3<391::AID-ANGE391>3.0.CO;2-M Google ScholarAngew. Chem. Int. Ed. 1999, 38, 379.10.1002/(SICI)1521-3773(19990201)38:3<379::AID-ANIE379>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 24
- 24aP. Rocca, C. Cochennec, F. Marsais, L. Thomas-dit-Dumont, A. Godard, G. Quéguiner, J. Org. Chem. 1993, 58, 7832;
- 24bC. Cochonnec, P. Rocca, F. Marsais, A. Godard, G. Quéguiner, Synthesis 1995, 321;
10.1055/s-1995-3896 Google Scholar
- 24cF. Trécourt, B. Gervais, M. Mallet, G. Quéguiner, J. Org. Chem. 1996, 61, 1673.
- 25
- 25aM. Lysén, J. L. Kristensen, P. Vedso, M. Begtrup, Org. Lett. 2002, 4, 257;
- 25bJ. L. Kristensen, M. Lysén, P. Vedso, M. Begtrup, Org. Lett. 2001, 3, 1435.
- 26
- 26aR. Chinchilla, C. Najera, M. Yus, Chem. Rev. 2004, 104, 2667;
- 26bF. D. Therkelsen, M. Rottländer, N. Thorup, E. Bjerregaard Pedersen, Org. Lett. 2004, 6, 1991.
- 27This sulfoxide/magnesium exchange has to be performed at −90 °C. At higher temperatures, a competitive radical process leads to extensive amounts of protonated species.
- 28
- 28aM. Rambaud, J. Villiéras, Synthesis 1984, 406;
- 28bJ. Villiéras, M. Rambaud, Org. Synth. 1988, 66, 220.
- 29To avoid extensive protonations (see Ref. [27]) the Grignard reagent obtained from compound 14 a had to be kept at low temperatures (−90 °C), which precludes effective quenching reactions with most electrophiles.
- 30
- 30aE. Negishi, A. O. King, N. Okukado, J. Org. Chem. 1977, 42, 1821;
- 30bE. Negishi, Acc. Chem. Res. 1982, 15, 340;
- 30cE. Negishi, L. F. Valente, M. Kobayashi, J. Am. Chem. Soc. 1980, 102, 3298.
- 31L. Jin, A. Lei, Org. Biomol. Chem. 2012, 10, 6817.
- 32A direct zinc insertion into the ClC bond of 21 did not lead to a 2-zincated azaindole derivative.
- 33
- 33aE. Mazri, C. Bobbio, F. Cottet, M. Schlosser, Eur. J. Org. Chem. 2005, 2116;
- 33bC. Bobbio, T. Rausis, M. Schlosser, Chem. Eur. J. 2005, 11, 1903;
- 33cF. Alonso, I. P. Beletskaya, M. Yus, Chem. Rev. 2002, 102, 4009.