Catalytic 1,4-Selective Hydrosilylation of Pyridines and Benzannulated Congeners†
Dipl.-Chem. C. David F. Königs
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin (Germany) http://www.organometallics.tu-berlin.de
Search for more papers by this authorDr. Hendrik F. T. Klare
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin (Germany) http://www.organometallics.tu-berlin.de
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Oestreich
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin (Germany) http://www.organometallics.tu-berlin.de
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin (Germany) http://www.organometallics.tu-berlin.de===Search for more papers by this authorDipl.-Chem. C. David F. Königs
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin (Germany) http://www.organometallics.tu-berlin.de
Search for more papers by this authorDr. Hendrik F. T. Klare
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin (Germany) http://www.organometallics.tu-berlin.de
Search for more papers by this authorCorresponding Author
Prof. Dr. Martin Oestreich
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin (Germany) http://www.organometallics.tu-berlin.de
Institut für Chemie, Technische Universität Berlin, Strasse des 17. Juni 115, 10623 Berlin (Germany) http://www.organometallics.tu-berlin.de===Search for more papers by this authorThis research was supported by the Technische Universität Berlin. M.O. is indebted to the Einstein Foundation (Berlin) for an endowed professorship.
Graphical Abstract
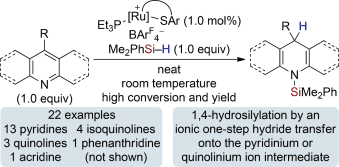
Radically different! The hydrosilylation of pyridines and quinolines is strictly 1,4-selective and likely involves an ionic one-step rather than the established radical two-step hydride transfer from a ruthenium(II) hydride complex onto the respective pyridinium and quinolinium ion intermediates (see scheme; ArF=3,5-(CF3)2C6H3). Even 4-substituted substrates react highly regioselectively. Isoquinolines yield the 1,2-reduced heterocycles.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201305028_sm_miscellaneous_information.pdf2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1N. Pollak, C. Dölle, M. Ziegler, Biochem. J. 2007, 402, 205–218.
- 2For reviews on the chemistry of dihydropyridines, see:
- 2aU. Eisner, J. Kuthan, Chem. Rev. 1972, 72, 1–42;
- 2bD. M. Stout, A. I. Meyers, Chem. Rev. 1982, 82, 223–243;
- 2cR. Lavilla, J. Chem. Soc. Perkin Trans. 1 2002, 1141–1156.
- 3For a review, see:
- 3aS. G. Ouellet, A. M. Walji, D. W. C. MacMillan, Acc. Chem. Res. 2007, 40, 1327–1339; for seminal original contributions, see:
- 3bM. Rueping, E. Sugiono, C. Azap, T. Theissmann, M. Bolte, Org. Lett. 2005, 7, 3781–3783;
- 3cS. Hoffmann, A. M. Seayad, B. List, Angew. Chem. 2005, 117, 7590–7593;
10.1002/ange.200503062 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 7424–7427;
- 3dR. I. Storer, D. E. Carrera, Y. Ni, D. W. C. MacMillan, J. Am. Chem. Soc. 2006, 128, 84–86.
- 4
- 4aJ. G. Keay, Adv. Heterocycl. Chem. 1986, 39, 1–77;
- 4bJ. G. Keay in Comprehensive Organic Synthesis, Vol. 8 (Eds.: ), Pergamon, Oxford, 1991, pp. 579–602.
- 5For a review discussing unconventional ways to regenerate 1,4-dihydropyridines from pyridinium ions, see: F. Hollmann, I. W. C. E. Arends, K. Buehler, ChemCatChem 2010, 2, 762–782.
- 6
- 6aB. D. Shaw, J. Chem. Soc. 1925, 215–216;
- 6bB. D. Shaw, J. Chem. Soc. 1937, 300–302;
- 6cA. J. Birch, J. Chem. Soc. 1947, 1270;
- 6dS. Danishefsky, A. Nagel, J. Chem. Soc. Chem. Commun. 1972, 373.
- 7A. J. Birch, E. A. Karakhanov, J. Chem. Soc. Chem. Commun. 1975, 480–481.
- 8
- 8aT. J. Donohoe, A. J. McRiner, P. Sheldrake, Org. Lett. 2000, 2, 3861–3863;
- 8bT. J. Donohoe, A. J. McRiner, M. Helliwell, P. Sheldrake, J. Chem. Soc. Perkin Trans. 1 2001, 1435–1445.
- 9
- 9aA. P. Shaw, B. L. Ryland, M. J. Franklin, J. R. Norton, J. Y.-C. Chen, M. Lynn Hall, J. Org. Chem. 2008, 73, 9668–9674 (one example of a ruthenium(II)-catalyzed 1,4-reduction of an acyl pyridinium ion, 58 % conv.);
- 9bQ.-A. Chen, M.-W. Chen, C.-B. Yu, L. Shi, D.-S. Wang, Y. Yang, Y.-G. Zhou, J. Am. Chem. Soc. 2011, 133, 16432–16435 (one example of a ruthenium(II)-catalyzed 1,4-reduction of a Hantzsch ester, 69 % conv.).
- 10M. Arrowsmith, M. S. Hill, T. Hadlington, G. Kociok-Köhn, C. Weetman, Organometallics 2011, 30, 5556–5559.
- 11K. Oshima, T. Ohmura, M. Suginome, J. Am. Chem. Soc. 2012, 134, 3699–3702.
- 12Suginome and co-workers also reported a palladium(II)-catalyzed silaboration of pyridines, affording 1,4- or 1,2-dihydropyridines depending on the substitution pattern: K. Oshima, T. Ohmura, M. Suginome, J. Am. Chem. Soc. 2011, 133, 7324–7327.
- 13For the heterogeneous hydrosilylation of pyridines, see:
- 13aN. C. Cook, J. E. Lyons, J. Am. Chem. Soc. 1965, 87, 3283–3284;
- 13bN. C. Cook, J. E. Lyons, J. Am. Chem. Soc. 1966, 88, 3396–3403.
- 14
- 14aL. Hao, J. F. Harrod, A.-M. Lebuis, Y. Mu, R. Shu, E. Samuel, H.-G. Woo, Angew. Chem. 1998, 110, 3314–3318;
10.1002/(SICI)1521-3757(19981116)110:22<3314::AID-ANGE3314>3.0.CO;2-G Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 3126–3129;10.1002/(SICI)1521-3773(19981204)37:22<3126::AID-ANIE3126>3.0.CO;2-Q CAS Web of Science® Google Scholar
- 14bJ. F. Harrod, R. Shu, H.-G. Woo, E. Samuel, Can. J. Chem. 2001, 79, 1075–1085.
- 15
- 15aD. V. Gutsulyak, A. van der Est, G. I. Nikonov, Angew. Chem. 2011, 123, 1420–1423; Angew. Chem. Int. Ed. 2011, 50, 1384–1387; for a highlight article, see:
- 15bK. Osakada, Angew. Chem. 2011, 123, 3929–3930; Angew. Chem. Int. Ed. 2011, 50, 3845–3846.
- 16Y. Ohki, Y. Takikawa, H. Sadohara, C. Kesenheimer, B. Engendahl, E. Kapatina, K. Tatsumi, Chem. Asian J. 2008, 3, 1625–1635.
- 17
- 17aH. F. T. Klare, M. Oestreich, J.-i. Ito, H. Nishiyama, Y. Ohki, K. Tatsumi, J. Am. Chem. Soc. 2011, 133, 3312–3315;
- 17bC. D. F. Königs, H. F. T. Klare, Y. Ohki, K. Tatsumi, M. Oestreich, Org. Lett. 2012, 14, 2842–2845;
- 17cC. D. F. Königs, M. F. Müller, N. Aiguabella, H. F. T. Klare, M. Oestreich, Chem. Commun. 2013, 49, 1506–1508;
- 17dT. Stahl, H. F. T. Klare, M. Oestreich, J. Am. Chem. Soc. 2013, 135, 1248–1251.
- 18For a review addressing this issue, see: I. S. Poddubnyi, Khim. Geterotsikl. Soedin. 1995, 774–815 (Chem. Heterocycl. Compd. 1995, 31, 682–714), and references therein.
- 19A. M. Voutchkova, D. Gnanamgari, C. E. Jakobsche, C. Butler, S. J. Miller, J. Parr, R. H. Crabtree, J. Organomet. Chem. 2008, 693, 1815–1821.
- 20S. J. Geier, P. A. Chase, D. W. Stephan, Chem. Commun. 2010, 46, 4884–4886.
- 21
- 21aD. Griller, K. U. Ingold, Acc. Chem. Res. 1980, 13, 317–323;
- 21bM. Newcomb, Tetrahedron 1993, 49, 1151–1176.