Visible-Light Hypervalent Iodide Carboxylate Photo(trifluoro)methylations and Controlled Radical Polymerization of Fluorinated Alkenes†
Corresponding Author
Prof. Alexandru D. Asandei
Department of Chemistry and Institute of Materials Science, University of Connecticut, 97 N. Eagleville Rd. Storrs, CT, 06269-3136 (USA)
Department of Chemistry and Institute of Materials Science, University of Connecticut, 97 N. Eagleville Rd. Storrs, CT, 06269-3136 (USA)===Search for more papers by this authorOlumide I. Adebolu
Department of Chemistry and Institute of Materials Science, University of Connecticut, 97 N. Eagleville Rd. Storrs, CT, 06269-3136 (USA)
Search for more papers by this authorChristopher P. Simpson
Department of Chemistry and Institute of Materials Science, University of Connecticut, 97 N. Eagleville Rd. Storrs, CT, 06269-3136 (USA)
Search for more papers by this authorJoon-Sung Kim
Department of Chemistry and Institute of Materials Science, University of Connecticut, 97 N. Eagleville Rd. Storrs, CT, 06269-3136 (USA)
Search for more papers by this authorCorresponding Author
Prof. Alexandru D. Asandei
Department of Chemistry and Institute of Materials Science, University of Connecticut, 97 N. Eagleville Rd. Storrs, CT, 06269-3136 (USA)
Department of Chemistry and Institute of Materials Science, University of Connecticut, 97 N. Eagleville Rd. Storrs, CT, 06269-3136 (USA)===Search for more papers by this authorOlumide I. Adebolu
Department of Chemistry and Institute of Materials Science, University of Connecticut, 97 N. Eagleville Rd. Storrs, CT, 06269-3136 (USA)
Search for more papers by this authorChristopher P. Simpson
Department of Chemistry and Institute of Materials Science, University of Connecticut, 97 N. Eagleville Rd. Storrs, CT, 06269-3136 (USA)
Search for more papers by this authorJoon-Sung Kim
Department of Chemistry and Institute of Materials Science, University of Connecticut, 97 N. Eagleville Rd. Storrs, CT, 06269-3136 (USA)
Search for more papers by this authorThe University of Connecticut is acknowledged for support.
Graphical Abstract
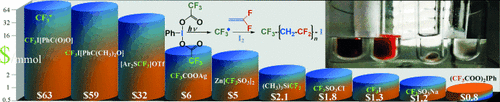
IFAB-ulous trifluoromethylation: (CX3COO)2IIIIPh (X=F, H) and (CH3COO)3IV(C6H4COO) are introduced as CX3./CX3I precursors for metal-free, visible-light, radical (trifluoro)(iodo)methylations of alkenes, illustrated by their use as photoinitiators for the controlled radical polymerization of vinylidene fluoride with external (I(CF2)6I) and in situ generated (CF3I) iodine chain transfer agents, and for block copolymer synthesis.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201303826_sm_miscellaneous_information.pdf3.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. A. McClinton, D. A. McClinton, Tetrahedron 1992, 48, 6555.
- 2J. A. Ma, D. J. Cahard, J. Fluorine Chem. 2007, 128, 975.
- 3T. Besset, C. Schneider, D. Cahard, Angew. Chem. 2012, 124, 5134;
10.1002/ange.201201012 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 5048.
- 4T. Furuya, A. S. Kamlet, T. Ritter, Nature 2011, 473, 470.
- 5X.-F. Wu, H. Neumann, M. R. Beller, Chem. Asian J. 2012, 7, 1744.
- 6S. Roy, B. T. Gregg, G. W. Gribble, V. D. Le, S. Roy, Tetrahedron 2011, 67, 2161.
- 7G. K. S. Prakash, P. V. Jog, T. D. Patrice, P. T. D. Batamack, G. A. Olah, Science 2012, 338, 1324.
- 8E. Mejía, A. Togni, ACS Catal. 2012, 2, 521.
- 9O. A. Tomashenko, V. V. Grushin, Chem. Rev. 2011, 111, 4475.
- 10A. Studer, Angew. Chem. 2012, 124, 9082; Angew. Chem. Int. Ed. 2012, 51, 8950.
- 11S. Mizuta, S. Verhoog, K. M. Engle, T. Khotavivattana, M. O’Duill, K. Wheelhouse, G. Rassias, M. Médebielle, V. Gouverneur, J. Am. Chem. Soc. 2013, 135, 2505.
- 12C.-J. Wallentin, J. D. Nguyen, P. Finkbeiner, R. J. Corey, C. R. J. Stephenson, J. Am. Chem. Soc. 2012, 134, 8875.
- 13M. G. Dhara, S. Banerjee, Prog. Polym. Sci. 2010, 35, 1022.
- 142013 Prices ($ per mmol CF3*, Sigma-Aldrich): CF3I[C6H4C(O)O], 63.2; CF3I[C6H4C(CH3)2O], 59.4; [Ar2SCF3]OTf, 32.4; CF3COOAg, 5.91; (CF3SO2)2Zn, 4.53; TMS-CF3 2.1; CF3-SO2-Cl, 1.76; CF3-I, 1.35; CF3-SO2-Na, 1.40; (CF3COO)2IPh 0.81.
- 15B. Ameduri, Chem. Rev. 2009, 109, 6632.
- 16B. Ameduri, B. Boutevin, Well Architectured Fluoropolymers: Synthesis Properties and Applications, Elsevier, Amsterdam 2004, pp. 1–99.
10.1016/B978-008044388-1/50010-8 Google Scholar
- 17W. A. Braunecker, K. Matyjaszewski, Prog. Polym. Sci. 2007, 32, 93.
- 18A. Goto, T. Fukuda, Prog. Polym. Sci. 2004, 29, 329.
- 19A. D. Asandei, O. I. Adebolu, C. P. Simpson, J. Am. Chem. Soc. 2012, 134, 6080.
- 20M. Oka, M. Tatemoto, Contemporary Topics in Polymer Science, Vol. 4, Plenum, New York, 1984, p. 763.
10.1007/978-1-4615-6743-1_49 Google Scholar
- 21G. David, C. Boyer, J. Tonnar, B. Ameduri, P. Lacroix-Desmazes, B. Boutevin, Chem. Rev. 2006, 106, 3936.
- 22A. D. Asandei, C. P. Simpson, O. I. Adebolu, ACS Symp. Ser. 2012, 1106, 47.
- 23S. Yamago, Y. Nakamura, Polymer 2013, 54, 981–994.
- 24P. J. Stang, V. V. Zhdankin, Chem. Rev. 1996, 96, 1123.
- 25P. J. Stang, V. V. Zhdankin, Chem. Rev. 2002, 102, 2523.
- 26P. J. Stang, V. V. Zhdankin, Chem. Rev. 2008, 108, 5299.
- 27H. Togo, M. Katohgia, Synlett 2001, 565.
- 28H. Suginome, CRC Handbook of Organic Photochemistry and Photobiology, CRC, Boca Raton, 2004, pp. 109-1–44.
- 29J. L. Courtneidge, J. Lusztyk, D. Page, Tetrahedron Lett. 1994, 35, 1003.
- 30J. Madsen, C. Viuf, M. Bols, Chem. Eur. J. 2000, 6, 1140.
10.1002/(SICI)1521-3765(20000403)6:7<1140::AID-CHEM1140>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 31A. Alberti, M. Benaglia, Res. Chem. Intermed. 1989, 11, 117.
- 32T. Kitamura, CRC Handbook of Organic Photochemistry and Photobiology, CRC, Boca Raton, 2004, pp. 110-1–11.
- 33L. M. Richards, U. S. Patent 2,467,231, 1949.
- 34G. S. Georgiev, Polym. Bull. 1999, 43, 223.
- 35H. Han, N. V. Tsarevsky, Polym. Chem. 2012, 3, 1910.
- 36R. Kopitzky, H. Willner, A. Hermann, H. Oberhammer, Inorg. Chem. 2001, 40, 2693.
- 37T. P. Yoon, M. A. Ischay, J. Du, Nat. Chem. 2010, 2, 527.
- 38W. D. Johnson, J. E. Sherwood, Aust. J. Chem. 1971, 24, 2281.
- 39R. Kaptein, J. Brokken-Zijp, F. J. J. de Kanter, J. Am. Chem. Soc. 1972, 94, 6280.
- 40V. D. Filimonov, O. K. Poleshchuk, E. A. Krasnokutskaya, G. Frenking, J. Mol. Model. 2011, 17, 2759.
- 41P. Lacroix-Desmazes, J. Tonnar, B. Boutevin, Macromol. Symp. 2007, 248, 150.
- 42H. Gottam, T. K. J. Vinod, Org. Chem. 2011, 76, 974.
- 43J. Gallos, A. J. Varvoglis, Chem. Soc. Perkin Trans. 1 1983, 1999.
- 44M. R. Britten-Kelley, A. Goosen, A. Scheffer, J. South African Chem. Inst. 1975, 28, 224.
- 45F. Fontana, F. Minisci, M. C. N. Barbosa, E. Vismara, Gazz. Chim. Ital. 1992, 122, 167.
- 46P. Gray, A. A. Herod, A. Jones, Chem. Rev. 1971, 71, 247.
- 47J. A. LaVerne, L. Wojnarovits, J. Phys. Chem. 1994, 98, 12635.
- 48M. Hauptschein, R. E. Oesterling, M. Braid, E. A. Tyczkowski, D. M. Gardner, J. Org. Chem. 1963, 28, 1281.