The 2-Phosphaethynolate Anion: A Convenient Synthesis and [2+2] Cycloaddition Chemistry†
Andrew R. Jupp
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Search for more papers by this authorCorresponding Author
Dr. Jose M. Goicoechea
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx===Search for more papers by this authorAndrew R. Jupp
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Search for more papers by this authorCorresponding Author
Dr. Jose M. Goicoechea
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx===Search for more papers by this authorWe thank the EPSRC and the University of Oxford for financial support of this research (DTA studentship to A.R.J.) and the University of Oxford for access to Chemical Crystallography and OSC facilities. We also thank Elemental Microanalysis Ltd. (Devon) for performing the elemental analyses. R. S. P. Turbervill is acknowledged for the synthesis of the ketene and carbodiimide.
Graphical Abstract
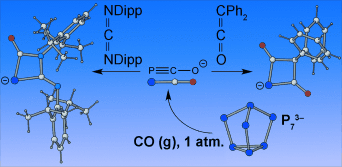
Hip to be square: Direct carbonylation of solutions of the heptaphosphide trianion (P73−) afforded the phosphaethynolate anion in moderate yields. This species undergoes [2+2] cycloaddition reactions with diphenylketene and bis(2,6-diisopropylphenyl)carbodiimide to yield the anionic four-membered heterocycles P[C(O)]2C(C6H5)2− and PC(O)(CNDipp)NDipp−.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201305235_sm_miscellaneous_information.pdf913 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1G. Becker, W. Schwarz, N. Seidler, M. Westerhausen, Z. Anorg. Allg. Chem. 1992, 612, 72–82.
- 2M. Westerhausen, S. Schneiderbauer, H. Piotrowski, M. Suter, H. Nöth, J. Organomet. Chem. 2002, 643–644, 189–193.
- 3F. F. Puschmann, D. Stein, D. Heift, C. Hendriksen, Z. A. Gal, H.-F. Grützmacher, H. Grützmacher, Angew. Chem. 2011, 123, 8570–8574;
10.1002/ange.201102930 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 8420–8423.
- 4I. Krummenacher, C. C. Cummins, Polyhedron 2012, 32, 10–13.
- 5
- 5aR. S. P. Turbervill, A. R. Jupp, P. S. B. McCullough, D. Ergöçmen, J. M. Goicoechea, Organometallics 2013, 32, 2234–2244;
- 5bR. S. P. Turbervill, J. M. Goicoechea, Inorg. Chem. 2013, 52, 5527–5534;
- 5cR. S. P. Turbervill, J. M. Goicoechea, Organometallics 2012, 31, 2452–2462;
- 5dR. S. P. Turbervill, J. M. Goicoechea, Chem. Commun. 2012, 48, 1470–1472;
- 5eR. S. P. Turbervill, J. M. Goicoechea, Chem. Commun. 2012, 48, 6100–6102.
- 6Full experimental details and characterization data for all of the compounds reported herein are available in the Supporting Information.
- 7
- 7aM. Baudler, D. Düster, K. Langerbeins, J. Germeshausen, Angew. Chem. 1984, 96, 309–310; Angew. Chem. Int. Ed. Engl. 1984, 23, 317–318;
- 7bM. Baudler, Angew. Chem. 1982, 94, 520–539; Angew. Chem. Int. Ed. Engl. 1982, 21, 492–512.
- 8CCDC 944181 ([K([18]crown-6)]-1), CCDC 944182 ([K([18]crown-6)]-2), and CCDC 944183 ([K([18]crown-6)]-3) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 9
- 9aC. Jones, M. Waugh, J. Organomet. Chem. 2007, 692, 5086–5090;
- 9bJ. G. Cordaro, D. Stein, H. Grützmacher, J. Am. Chem. Soc. 2006, 128, 14962–14971;
- 9cK. Toyota, S. Kawasaki, M. Yoshifuji, J. Org. Chem. 2004, 69, 5065–5070;
- 9dM. Y. Antipin, A. N. Chernega, K. A. Lysenko, Y. T. Struchkov, J. F. Nixon, J. Chem. Soc. Chem. Commun. 1995, 505–506;
- 9eA. N. Chernega, M. Y. Antipin, Y. T. Struchkov, M. F. Meidine, J. F. Nixon, Heteroat. Chem. 1991, 2, 665–667;
- 9fA. M. Arif, A. R. Barron, A. H. Cowley, S. W. Hall, J. Chem. Soc. Chem. Commun. 1988, 171–172.
- 10
- 10aL. Weber, B. Torwiehe, G. Bassmann, H.-G. Stammler, B. Neumann, Organometallics 1996, 15, 128–132;
- 10bG. Becker, G. Heckmann, K. Hubler, W. Schwarz, Z. Anorg. Allg. Chem. 1995, 621, 34–46.
- 11S. Alidori, D. Heift, G. Santiso-Quinones, Z. Benkö, H. Grützmacher, M. Caporali, L. Gonsalvi, A. Rossin, M. Peruzzini, Chem. Eur. J. 2012, 18, 14805–14811.
- 12
- 12aK. B. Dillon, F. Mathey, J. F. Nixon, Phosphorus: The Carbon Copy: From Organophosphorus to Phospha-organic Chemistry, 1st ed., Wiley, Chichester, 1998;
- 12bJ. F. Nixon, Chem. Rev. 1988, 88, 1327–1362;
- 12cF. Mathey, Coord. Chem. Rev. 1994, 137, 1–52;
- 12dJ. F. Nixon, Chem. Soc. Rev. 1995, 24, 319–328;
- 12eP. Le Floch, F. Mathey, Coord. Chem. Rev. 1998, 178, 771–791;
- 12fF. Mathey, Angew. Chem. 2003, 115, 1616–1643;
10.1002/ange.200200557 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1578–1604.
- 13
- 13aL. Weber, K. Relzig, R. Boese, Organometallics 1985, 4, 1890–1891.
- 14
- 14aP. Pyykkö, M. Atsumi, Chem. Eur. J. 2009, 15, 186–197;
- 14bP. Pyykkö, M. Atsumi, Chem. Eur. J. 2009, 15, 12770–12779.
- 15C. L. Liotta, M. L. McLaughlin, D. G. V. Derveer, B. A. O’Brien, Tetrahedron Lett. 1984, 25, 1665–1668.
- 16
- 16aL. Weber, S. Kleinebekel, L. Pumpenmeier, H.-G. Stammler, B. Neumann, Organometallics 2002, 21, 1998–2005;
- 16bL. Weber, B. Quasdorff, H.-G. Stammler, B. Neumann, Chem. Eur. J. 1998, 4, 469–475;
- 16cY. W. Li, M. G. Newton, R. B. King, Inorg. Chem. 1993, 32, 5720–5729;
- 16dL. Weber, S. Buchwald, D. Lentz, O. Stamm, D. Preugschat, R. Marschall, Organometallics 1994, 13, 4406–4412.
- 17M. Sebastian, M. Nieger, D. Szieberth, L. Nyulászi, E. Niecke, Angew. Chem. 2004, 116, 647–651;
10.1002/ange.200352746 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 637–641.
- 18V. Lyaskovskyy, N. Elders, A. W. Ehlers, M. Lutz, J. C. Slootweg, K. Lammertsma, J. Am. Chem. Soc. 2011, 133, 9704–9707.
- 19For selected reports, see:
- 19aP. B. Hitchcock, M. J. Maah, J. F. Nixon, Chem. Commun. 1986, 737–738;
- 19bT. Wettling, G. Wolmershäuser, P. Binger, M. Regitz, Chem. Commun. 1990, 1541–1543;
- 19cJ. J. Schneider, U. Denninger, O. Heinemann, C. Krüger, Angew. Chem. 1995, 107, 631–634;
10.1002/ange.19951070520 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 592–595;
- 19dR. Wolf, A. W. Ehlers, J. C. Slootweg, M. Lutz, D. Gudat, M. Hunger, A. L. Spek, K. Lammertsma, Angew. Chem. 2008, 120, 4660–4663; Angew. Chem. Int. Ed. 2008, 47, 4584–4587;
- 19eR. Wolf, J. C. Slootweg, A. W. Ehlers, F. Hartl, B. de Bruin, M. Lutz, A. L. Spek, K. Lammertsma, Angew. Chem. 2009, 121, 3150–3153; Angew. Chem. Int. Ed. 2009, 48, 3104–3107;
- 19fR. Wolf, E.-M. Schnöckelborg, Chem. Commun. 2010, 46, 2832–2834.
- 20For selected reports, see:
- 20aC. L. Mandell, S. S. Kleinbach, W. G. Dougherty, W. S. Kassel, C. Nataro, Inorg. Chem. 2010, 49, 9718–9727;
- 20bD. Coleman, P. G. Edwards, B. M. Kariuki, P. D. Newman, Dalton Trans. 2010, 39, 3842–3850;
- 20cB. K. Muñoz, E. Santos Garcia, C. Godard, E. Zangrando, C. Bo, A. Ruiz, C. Claver, Eur. J. Inorg. Chem. 2008, 4625–4637;
- 20dP. Pinto, A. W. Götz, G. Marconi, B. A. Hess, A. Marinetti, F. W. Heinemann, U. Zenneck, Organometallics 2006, 25, 2607–2616;
- 20eT. J. Brunker, N. F. Blank, J. R. Moncarz, C. Scriban, B. J. Anderson, D. S. Glueck, L. N. Zakharov, J. A. Golen, R. D. Sommer, C. D. Incarvito, A. L. Rheingold, Organometallics 2005, 24, 2730–2746;
- 20fJ. You, H.-J. Drexler, S. Zhang, C. Fischer, D. Heller, Angew. Chem. 2003, 115, 942–945; Angew. Chem. Int. Ed. 2003, 42, 913–916;
- 20gA. Marinetti, F. Labrue, B. Pons, S. Jus, L. Ricard, J.-P. Genêt, Eur. J. Inorg. Chem. 2003, 2583–2590;
- 20hD. C. R. Hockless, Y. B. Kang, M. A. McDonald, M. Pabel, A. C. Willis, S. B. Wild, Organometallics 1996, 15, 1301–1306;
- 20iA. Marinetti, C. Le Menn, L. Ricard, Organometallics 1995, 14, 4983–4985.