Syntheses and Structures of an “Alumole” and Its Dianion†
Dr. Tomohiro Agou
Institute for Chemical Research, Kyoto University, Gokasho, Uji, Kyoto 611-0011 (Japan)
Search for more papers by this authorTatsuya Wasano
Institute for Chemical Research, Kyoto University, Gokasho, Uji, Kyoto 611-0011 (Japan)
Search for more papers by this authorDr. Peng Jin
Fukui Institute for Fundamental Chemistry, Kyoto University, Kyoto 606-8103 (Japan)
Search for more papers by this authorProf. Dr. Shigeru Nagase
Fukui Institute for Fundamental Chemistry, Kyoto University, Kyoto 606-8103 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Norihiro Tokitoh
Institute for Chemical Research, Kyoto University, Gokasho, Uji, Kyoto 611-0011 (Japan)
Institute for Chemical Research, Kyoto University, Gokasho, Uji, Kyoto 611-0011 (Japan)===Search for more papers by this authorDr. Tomohiro Agou
Institute for Chemical Research, Kyoto University, Gokasho, Uji, Kyoto 611-0011 (Japan)
Search for more papers by this authorTatsuya Wasano
Institute for Chemical Research, Kyoto University, Gokasho, Uji, Kyoto 611-0011 (Japan)
Search for more papers by this authorDr. Peng Jin
Fukui Institute for Fundamental Chemistry, Kyoto University, Kyoto 606-8103 (Japan)
Search for more papers by this authorProf. Dr. Shigeru Nagase
Fukui Institute for Fundamental Chemistry, Kyoto University, Kyoto 606-8103 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Norihiro Tokitoh
Institute for Chemical Research, Kyoto University, Gokasho, Uji, Kyoto 611-0011 (Japan)
Institute for Chemical Research, Kyoto University, Gokasho, Uji, Kyoto 611-0011 (Japan)===Search for more papers by this authorThis work was partially supported by JSPS KAKENHI Grant (Nos. 22350017, 24550048, 24655028, and 24109013) and by the “Molecular Systems Research” project of RIKEN Advanced Science Institute. T.A. thanks to the Kyoto Technoscience Center for the financial support.
Graphical Abstract
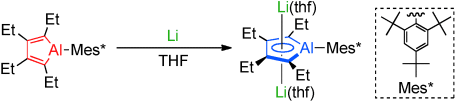
Base free: An alumole was synthesized and treatment with lithium afforded the lithium salt of the alumole dianion. The structures of these two molecules were then investigated. The CC bond lengths of the AlC4 ring in the dianion are nearly equal. DFT calculations revealed that the 3p(Al)–π* conjugation lowers the LUMO level of the alumole and that coordination of two lithium cations to the alumole dianion results in a planar AlC4 ring.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201304143_sm_miscellaneous_information.pdf612.3 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. Braunschweig, T. Kupfer, Chem. Commun. 2011, 47, 10903.
- 2H. Braunschweig, V. Dyakonov, J. O. C. Jimenez-Halla, K. Kraft, I. Krummenacher, K. Radacki, A. Sperlich, J. Wahler, Angew. Chem. 2012, 124, 3031; Angew. Chem. Int. Ed. 2012, 51, 2977.
- 3
- 3aJ. J. Eisch, J. E. Galle, S. Kozima, J. Am. Chem. Soc. 1986, 108, 379;
- 3bC.-W. So, D. Watanabe, A. Wakamiya, S. Yamaguchi, Organometallics 2008, 27, 3496;
- 3cH. Braunschweig, C.-W. Chiu, J. Wahler, K. Radacki, T. Kupfer, Chem. Eur. J. 2010, 16, 12229.
- 4Various aromaticity indexes, including nucleus-independent chemical shifts (NICS), harmonic oscillator model of aromaticity (HOMA), and aromatic stabilization energies, have been calculated for parent alumole 4; these indexes suggest that the antiaromaticity of 4 is much lower compared to that of the parent borole (HBC4H4). Similarly, the small positive NICS(0) value of 1 (+2.78 ppm) indicates the quite low antiaromaticity of 1;
- 4aP. von Ragué Schleyer, P. K. Freeman, H. Jiao, B. Goldfuss, Angew. Chem. 1995, 107, 332;
10.1002/ange.19951070309 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 337;
- 4bP. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao, N. J. R. van Eikema Hommes, J. Am. Chem. Soc. 1996, 118, 6317;
- 4cM. K. Cyrañsky, T. M. Krygowsky, A. R. Katritzky, P. v. R. Schleyer, J. Org. Chem. 2002, 67, 1333;
- 4dH. Fallah-Bagher-Shaidaei, C. S. Wannere, C. Corminboeuf, R. Puchta, P. v. R. Schleyer, Org. Lett. 2006, 8, 863;
- 4eD. B. Chesnut, L. J. Bartolotti, Chem. Phys. Lett. 2000, 316-331, 175;
- 4fJ. Poater, X. Fradera, M. Duran, M. Solà, Chem. Eur. J. 2003, 9, 400.
- 5Recently, syntheses and properties of dibenzogallole derivatives have been reported: T. Matsumoto, K. Tanaka, Y. Chujo, J. Am. Chem. Soc. 2013, 135, 4211.
- 6
- 6aH. Hoberg, R. Krause-Göing, J. Organomet. Chem. 1977, 127, C 29;
- 6bC. Krüger, J. C. Sekutowski, H. Hoberg, R. Krause-Göing, J. Organomet. Chem. 1977, 141, 141.
- 7Intermediary formation of alumoles:
- 7aE. Negishi, D. Y. Kondakov, D. Choueiry, K. Kasai, T. Takahashi, J. Am. Chem. Soc. 1996, 118, 9577;
- 7bZ. Xi, P. Li, Angew. Chem. 2000, 112, 3057;
Angew. Chem. Int. Ed. 2000, 39, 2950;
10.1002/1521-3773(20000818)39:16<2950::AID-ANIE2950>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 7cC. Zhao, P. Li, Z. Xi, Chem. Eur. J. 2002, 8, 4292;
10.1002/1521-3765(20020916)8:18<4292::AID-CHEM4292>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 7dJ. J. Eisch, W. C. Kaska, J. Am. Chem. Soc. 1962, 84, 1501;
- 7eJ. J. Eisch, W. C. Kaska, J. Am. Chem. Soc. 1966, 88, 2976;
- 7fH. Hoberg, W. Richter, J. Organomet. Chem. 1980, 195, 347.
- 8A. H. Cowley, F. P. Gabbaï, A. Decken, Angew. Chem. 1994, 106, 1429; Angew. Chem. Int. Ed. Engl. 1994, 33, 1370.
- 9A. Wakamiya, K. Mishima, K. Ekawa, S. Yamaguchi, Chem. Commun. 2008, 579.
- 10R. J. Wehmschulte, P. P. Power, Inorg. Chem. 1996, 35, 3262.
- 11L. Liu, W.-X. Zhang, Q. Luo, H. Li, Z. Xi, Organometallics 2010, 29, 278.
- 12The UV/Vis spectrum of 1 in n-hexane has an absorption at λmax=318 nm (ε=2.1×103), which is at a much shorter wavelength than those of the previously reported borole derivatives. The hypsochromic shift may be related with the much lower antiaromaticity of 1 compared to those of the borole derivatives. A relationship between the UV/Vis absorption properties and the antiaromaticity of pentaarylborole derivatives has been noted: H. Braunschweig, I. Fernández, G. Frenking, T. Kupfer, Angew. Chem. 2008, 120, 1977; Angew. Chem. Int. Ed. 2008, 47, 1951.
- 13Crystallographic data for 1: triclinic, space group P-1, a=11.3385(7), b=14.0940(13), c=19.1071(14) Å, α=70.677(4), β=88.960(3), γ=80.284(3)°, V=2837.7(4) Å3, Z=4, R1 (I>2σ(I))=0.0538, wR2 (all data)=0.1512; Crystallographic data for [Li+(thf)]2[12−]: tetragonal, space group P43212, a=b=10.4548(3), c=34.1514(10) Å, V=3732.9(2) Å3, Z=4, R1 (I>2σ (I))=0.0375, wR2 (all data)=0.1015. CCDC 915171 (1) and 915172 ([Li+(thf)]2[12−]) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 14W. Uhl, E. Er, A. Hepp, J. Kösters, J. Grunenberg, Organometallics 2008, 27, 3346.
- 15Reduction of a pentaphenylalumole/Et2O complex with lithium and subsequent treatment with NiBr2 afforded a unique triple-decker dinuclear nickel complex that does not contain aluminum: H. Hoberg, R. Krause-Göing, C. Krüger, J. C. Sekutowski, Angew. Chem. 1977, 89, 179; Angew. Chem. Int. Ed. Engl. 1977, 16, 183.
- 16The equilibrium structures of 42− and 12− are comparable to those of phospholes and heavier group 14 heterole anions. In the cases of these heteroles, the pyramidal forms and the planar forms correspond to the energy minima and the first-order saddle points on the potential surfaces, respectively:
- 16aW. P. Freeman, T. D. Tilley, F. P. Arnold, A. L. Rheingold, P. K. Gantzel, Angew. Chem. 1995, 107, 2029;
10.1002/ange.19951071724 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1887;
- 16bW. P. Freeman, T. D. Tilley, L. M. Liable-Sands, A. L. Rheingold, J. Am. Chem. Soc. 1996, 118, 10457;
- 16cL. Nyulászi, Chem. Rev. 2001, 101, 1229.
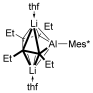
- 17A closo-7-vetex AlLi2C4 cluster description as shown below has been proposed for the bonding situation of [Li+(thf)]2[12−] by a reviewer. The cluster core consists of 16 skeletal electrons (3 electrons from each of the CEt units, 2 electrons from the AlMes* unit, and two negative charges), which agrees with the pentagonal bipyramidal structure as observed in the crystal and optimized structures of [Li+(thf)]2[12−].
- 18A. B. Pangborn, M. A. Giardello, R. H. Grubbs, R. K. Rosen, F. J. Timmers, Organometallics 1996, 15, 1518.
- 19Gaussian 09 (Revision C.01), M. J. Frisch, et al., Gaussian, Inc., Wallingford CT, 2010. For full reference, see Supporting Information.