Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture: Fluorous Synthesis of 18F Radiotracers with the [18F]Fluoride Ion: Nucleophilic Fluorination as the Detagging Process (Angew. Chem. Int. Ed. 3/2009)
- Page: 413
- First Published: 29 December 2008
![Cover Picture: Fluorous Synthesis of 18F Radiotracers with the [18F]Fluoride Ion: Nucleophilic Fluorination as the Detagging Process (Angew. Chem. Int. Ed. 3/2009)](/cms/asset/9270d9a7-5c80-40bb-856f-00538401961a/mcontent.jpg)
Time is of the essence in preparing 18F-labeled radiotracers when the half-life of 18F is 109.7 minutes. In their Communication on page 586, V. Gouverneur and co-workers report the radiosynthesis of various prosthetic groups and 18F radiotracers from fluorous-tagged precursors. This approach allows for fast and convenient purification of 18F-radiolabeled material (FSPE=fluorous solid phase extraction). Cover illustration produced by Dr. Karl Harrison (Chemistry Department, University of Oxford).
Inside Cover
Inside Cover: Robust Self-Assembly of Highly Ordered Complex Structures by Controlled Evaporation of Confined Microfluids (Angew. Chem. Int. Ed. 3/2009)
- Page: 414
- First Published: 29 December 2008

“Coffee rings” of different forms and sizes can be specifically “synthesized” as Z. Lin and co-workers report in their Communication on p. 512. Polymer solutions are confined in a space between a curved surface and a flat substrate. By using different geometrically curved surfaces, the controlled evaporation of the solution leads to self-assembly and the formation of higher-ordred structures over a large area.
Graphical Abstract
Graphical Abstract: Angew. Chem. Int. Ed. 3/2009
- Pages: 417-427
- First Published: 29 December 2008
News
Physical Organic Chemistry: H. Schwarz Honored / Carbohydrates: P. Seeberger Awarded / Organic Chemistry: Medal for H. Hopf
- Page: 430
- First Published: 29 December 2008
Spotlights on our sister journals: Angew. Chem. Int. Ed. 3/2009
- Pages: 432-433
- First Published: 29 December 2008
Book Reviews
Introduction to Modern Thermo dynamics. By Dilip Kondepudi.
- Page: 434
- First Published: 29 December 2008
Recent Developments in Carbocation and Onium Ion Chemistry. By Kenneth K. Laali.
- Page: 435
- First Published: 29 December 2008
Highlights
Fullerene Synthesis
One Step Closer to Isomerically Pure Fullerenes and Heterofullerenes: Harnessing the Potential of Catalytic Surfaces†
- Pages: 436-437
- First Published: 29 December 2008
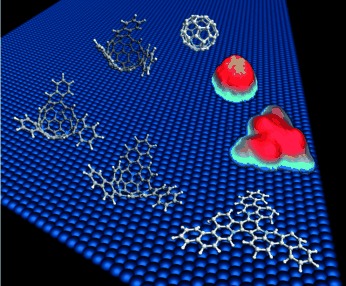
Fullerenes made to order: The ability of catalytic surfaces to stitch together the arms of polycyclic aromatic hydrocarbons and heterocycles to form fullerenes and heterofullerenes has been demonstrated. This ability may provide wide-ranging access to tailor-made fullerenes, with the specific arrangements of rings and heteroatoms dictated by the design and synthesis of the precursors.
Genetics
SLEEPLESS-ness and Insomnia in Fruit Flies†
- Pages: 438-440
- First Published: 29 December 2008

Lord of the flies: A gene, sleepless, has been identified in Drosophila that when mutagenized imparted an extreme short-sleeping phenotype. SLEEPLESS expression is independent of the circadian clock and was found to participate in the homeostatic regulation of sleep. Namely, SLEEPLESS appears to be a signaling molecule that couples sleep drive to changes in membrane excitability (see picture).
Minireview
Sustainable Chemistry
Aqueous Olefin Metathesis
- Pages: 442-454
- First Published: 29 December 2008

On and under water: Recent developments in aqueous olefin metathesis have followed two main strategies: 1) the use of water-insoluble commercially available ruthenium catalysts in aqueous mixtures and neat water, and 2) the design of special polar ruthenium catalysts that are active and stable in aqueous media. The general applicability of water as a solvent for olefin metathesis is shown.
Review
Chiral Catalysts
Nonlinear Effects in Asymmetric Catalysis
- Pages: 456-494
- First Published: 29 December 2008
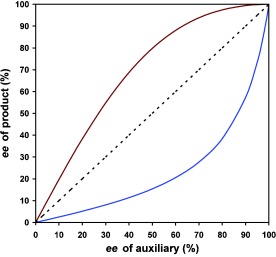
Informative deviation: The enantioselectivity (ee) of the reaction product arising from asymmetric catalysis with non-enantiopure chiral auxiliaries should be proportional to that of the chiral auxiliary or ligand (linearity). A deviation from linearity (nonlinear effects, see picture) can be rich in mechanistic information and can be synthetically useful (asymmetric amplification).
Communications
Asymmetric Synthesis
Stereocontrolled Solid-Phase Synthesis of Oligonucleoside H-Phosphonates by an Oxazaphospholidine Approach†
- Pages: 496-499
- First Published: 29 December 2008
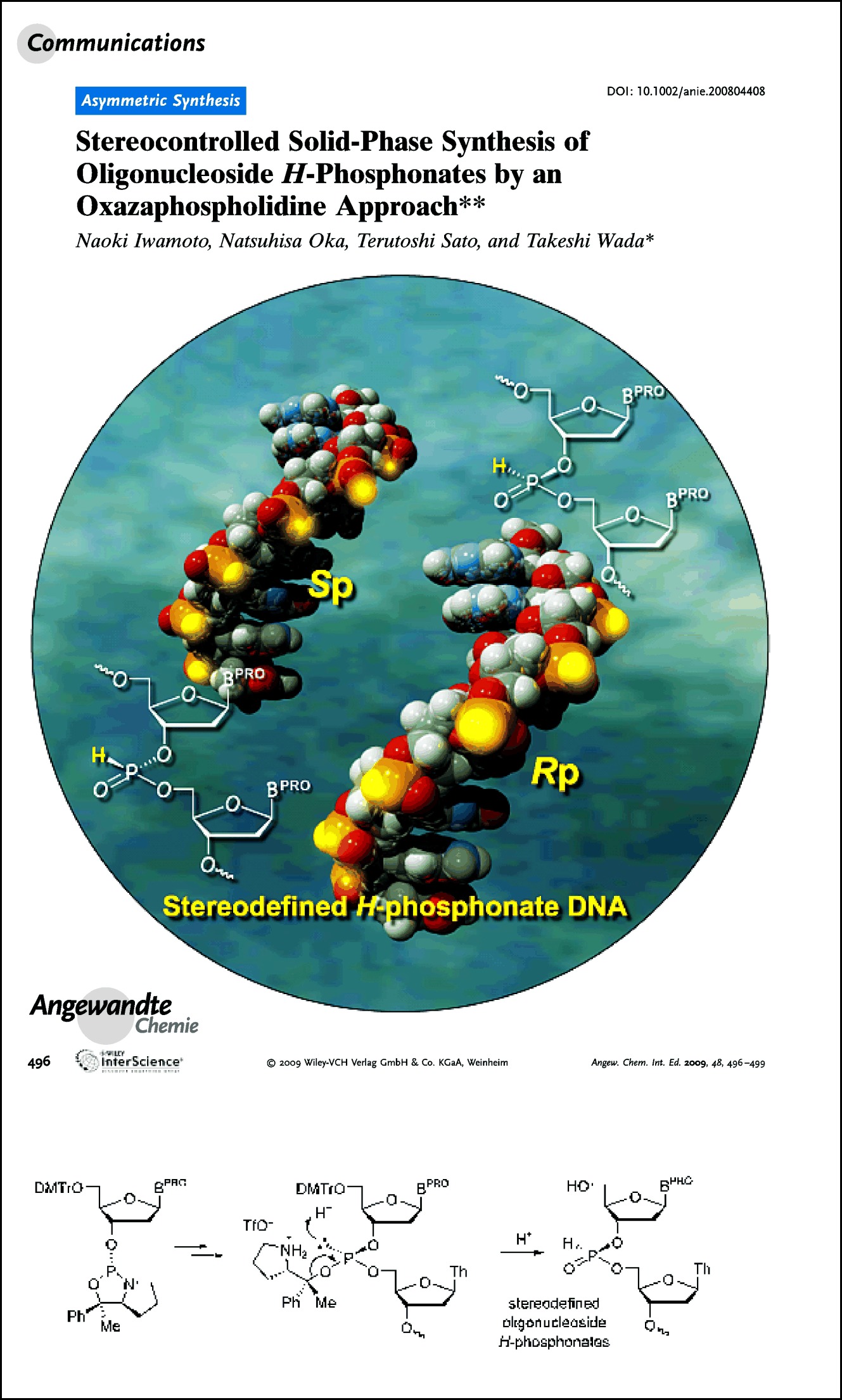
Stereodefined oligonucleoside H-phosphonates were synthesized on a solid support using diastereopure nucleoside 3′-O-oxazaphospholidine monomers. Several stereodefined backbone-modified analogues were obtained with the oligonucleoside H-phosphonates as precursors (see scheme; BPRO=protected nucleobase, DMTr=4,4′-dimethoxytrityl, Th=thymin-1-yl, TfO−=triflate).
Metal–Organic Frameworks
A Luminescent Metal–Organic Framework with Lewis Basic Pyridyl Sites for the Sensing of Metal Ions†
- Pages: 500-503
- First Published: 29 December 2008
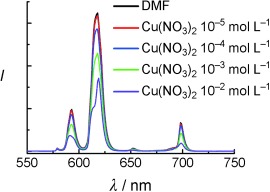
It all makes sense: A rationally developed europium metal–organic framework incorporating immobilized Lewis basic pyridyl sites oriented towards the center of one-dimensional channels is a rare example of a luminescent porous MOF. By comparison of the luminescence intensity quenching effects of various incorporated Lewis acidic metal cations, the MOF exhibits sensor capability.
Catenanes
Synthesis of [2]Catenanes by Oxidative Intramolecular Diyne Coupling Mediated by Macrocyclic Copper(I) Complexes†
- Pages: 504-507
- First Published: 29 December 2008
![Synthesis of [2]Catenanes by Oxidative Intramolecular Diyne Coupling Mediated by Macrocyclic Copper(I) Complexes](/cms/asset/16344e5f-7cf1-4c5e-bb87-54f4a0a95eef/mcontent.jpg)
Right said thread: Oxidative intramolecular coupling reactions of α,ω-diynes in the presence of macrocyclic phenanthroline CuI complexes allows the synthesis of [2]catenanes in up to 64 % yield (see scheme). The bond-forming reaction leads to concurrent threading of the diyne through the phenanthroline macrocycle.
Bacterial Networks
Self-Constructed Electrically Conductive Bacterial Networks†
- Pages: 508-511
- First Published: 29 December 2008
Concentric Patterns
Robust Self-Assembly of Highly Ordered Complex Structures by Controlled Evaporation of Confined Microfluids†
- Pages: 512-516
- First Published: 29 December 2008
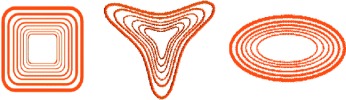
Coffee rings: Polymer solutions are confined in a simple geometry comprised of a curved surface placed upon a flat substrate. Simply by changing the shape of the upper surface of the imposed geometry, the controlled, evaporative self-assembly of polymer solutions yields a variety of complex, intriguing, and well-ordered structures over large areas (see picture).
Highly Strained Molecules
Isolation of Bicyclopropenylidenes: Derivatives of the Smallest Member of the Fulvalene Family†
- Pages: 517-520
- First Published: 29 December 2008
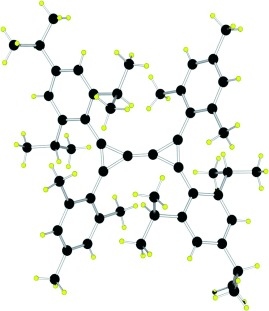
Small and stable: A triafulvalene derivative is indefinitely stable at room temperature both in solution and in the solid state under inert atmosphere. The triafulvalene is synthesized by the magnesium-catalyzed coupling of two bis(chlorocyclopropenyl) derivatives. The two unsaturated three-membered rings are linked together by a carbon–carbon double bond (1.303 Å, see structure), which is so reactive that spontaneous addition of water occurs at room temperature.
Single-Molecule Magnets
Enhancing the Quantum Properties of Manganese–Lanthanide Single-Molecule Magnets: Observation of Quantum Tunneling Steps in the Hysteresis Loops of a {Mn12Gd} Cluster†
- Pages: 521-524
- First Published: 29 December 2008
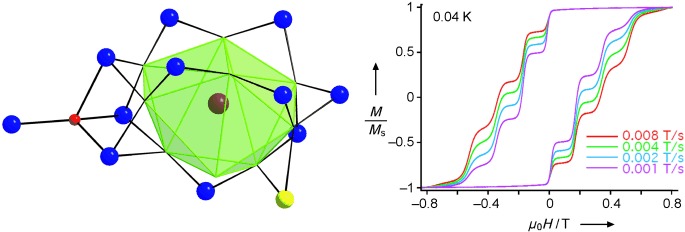
Quantum tunneling of magnetization (QTM) steps have been observed for a mixed 3d–4f single-molecule magnet for the first time in the hysteresis loops of {Mn12Gd}. This phenomenon is assigned to larger than usual exchange coupling of the 4f atom with the {Mn12} shell. This improved quantum behavior for the important class of SMMs opens up the prospects for its detailed study using hysteresis methods. Gd purple, Mn(II) yellow, Mn(III) blue, O red, N green.
Nano-biotechnology
A DNA Nanostructure for the Functional Assembly of Chemical Groups with Tunable Stoichiometry and Defined Nanoscale Geometry†
- Pages: 525-527
- First Published: 29 December 2008
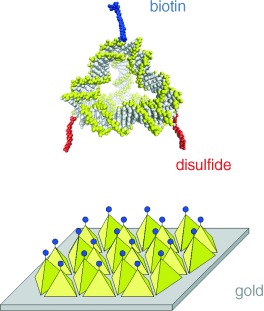
Many legs make light work: Tetrahedra are constructed with edges of double-stranded DNA and vertices tagged with biotin or disulfide units (see picture). They can act as supramolecular scaffolds to combine different chemical groups at defined nanoscale distances and with tunable stoichiometries. The disulfide groups bind to gold surfaces with high affinity, which leaves the biotin unit poised to capture streptavidin.
Electrochemical Cells
A Multiple Working Electrode for Electrochemical Cells: A Tool for Current Density Distribution Studies†
- Pages: 528-532
- First Published: 29 December 2008
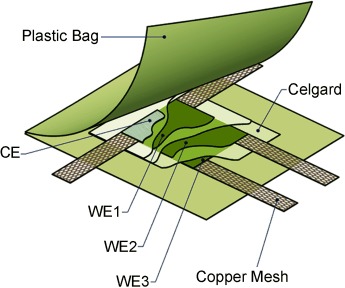
Uneven distribution: The multiple working electrode for electrochemical cells (see image) is a unique tool for the quantitative study of current density distribution in lithium-ion batteries. During the cycling of the cell even at a low C-rate of C/37, an inhomogeneity in the average current density distribution of more than 8 % is observed. WE=working electrode, CE=counter electrode.
Sorption Enhancement
Enhancement of Sorption Processes in the Zeolite H-ZSM5 by Postsynthetic Surface Modification†
- Pages: 533-538
- First Published: 29 December 2008
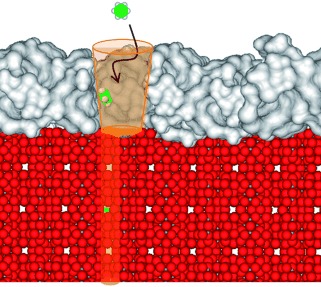
Catching molecules: When the surface of zeolite HZSM-5 (see picture, red) is modified by amorphous SiO2 layers (gray), increased surface roughness results. The mesopores in the rough SiO2 layer funnel benzene molecules into the micropores of the zeolite, thus increasing sorption rates for benzene at the acidic hydroxy groups by a factor of two. The size of the mesopores relative to the adsorbing molecules is critical for sorption-rate enhancement.
Chiral Catalysts
Helical Poly(quinoxaline-2,3-diyl)s Bearing Metal-Binding Sites as Polymer-Based Chiral Ligands for Asymmetric Catalysis†
- Pages: 539-542
- First Published: 29 December 2008
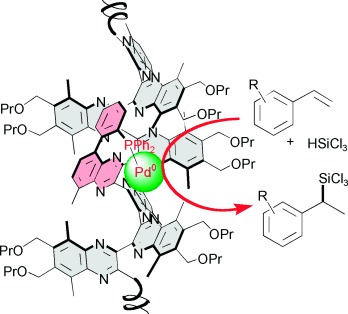
Living it up: Helical polyquinoxalines with single and multiple metal-binding sites, prepared by living polymerization of o-diisocyanobenzenes, are used in the asymmetric hydrosilylation of styrenes, resulting in comparable enantioselectivities to those obtained by low-molecular-weight catalyst systems (up to 87 % ee, stereochemistry was determined by a chiral initiator) and a turnover number of almost 1000.
Defect Chemistry
Orientation-Dependent Arrangement of Antisite Defects in Lithium Iron(II) Phosphate Crystals†
- Pages: 543-546
- First Published: 29 December 2008
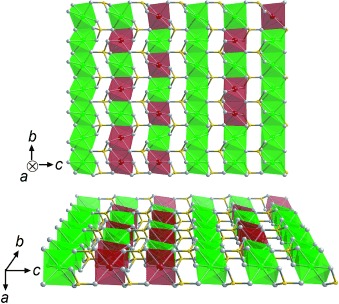
Aggregated antisite cations of iron (see picture, red) in the lithium sites of doped lithium iron phosphate (LiFePO4) are arranged preferentially along the b axis. To probe the peculiar array of the defects, Z-contrast STEM with a spherical-aberration correction is utilized. The images obtained using Z-contrast STEM suggest that the distribution of antisite defects in LiFePO4 can be adjusted for improved lithium ion transport.
Enantioselective Silylation
Catalytic Enantioselective Silylation of Acyclic and Cyclic Triols: Application to Total Syntheses of Cleroindicins D, F, and C†
- Pages: 547-550
- First Published: 29 December 2008
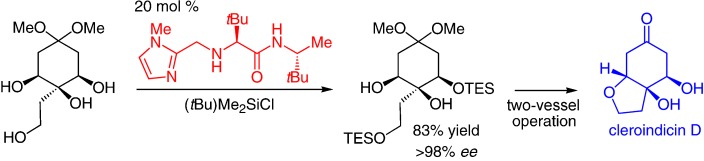
Pick one out of three: Acyclic and cyclic 1,2,3-triols are silylated with exceptional site- and enantioselectivity by a small-molecule catalyst to afford silyl ethers having a neighboring diol moiety. The new process is applied to the enantioselective total syntheses of three cleroindicins, natural products isolated from a plant used in China to battle malaria and rheumatism.
Homogeneous Catalysis
Self-Assembled Bidentate Ligands for the Nickel-Catalyzed Hydrocyanation of Alkenes†
- Pages: 551-554
- First Published: 29 December 2008
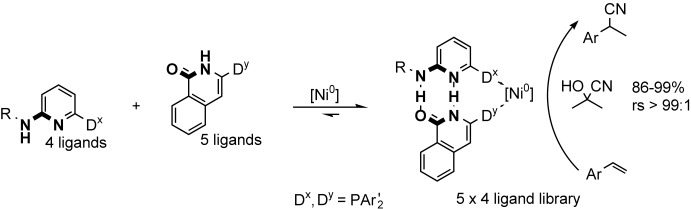
Well-stocked library: A catalyst with interesting activity, regioselectivity (rs), and functional-group tolerance could be identified from a 5×4 library of isoquinolone- or aminopyridine-derived self-assembled bidentate ligands. These ligands, which self-assemble through hydrogen bonding, form complexes with nickel(0) that appeared to be promising catalysts for the hydrocyanation of styrene (see scheme).
N-Heterocyclic Carbenes
A Simple Preparation of Pyridine-Derived N-Heterocyclic Carbenes and Their Transformation into Bridging Ligands by Orthometalation†
- Pages: 555-558
- First Published: 29 December 2008
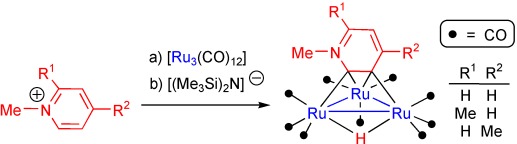
Pyrid-2-ylidenes are trapped in solution by [Ru3(CO)12] after being formed by deprotonation of N-substituted pyridinium cations. The great basicity of these NHC ligands and the polynuclear character of the ruthenium cluster trigger room temperature orthometalation of the initial κ1-C2-pyrid-2-ylidene ligands, leading to unprecedented face-capping κ2-C2,C3-pyrid-3-yl-2-ylidene ligands.
Synthetic Methods
Catalyzed Dehydrogenative Coupling of Primary Alcohols with Water, Methanol, or Amines†
- Pages: 559-563
- First Published: 29 December 2008
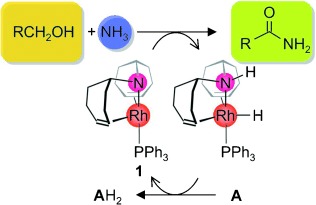
A working partnership: Metal–ligand cooperativity is responsible for the high activity of the rhodium amido complex 1 in the dehydrogenative coupling of primary alcohols with water, methanol, or amines, including ammonia (see scheme), to give carboxylic acids, methyl carboxylates, or amides, respectively. The catalysis proceeds under mild reaction conditions in the presence of a recyclable hydrogen acceptor A. The multistep mechanism was elucidated by computational methods.
Energetic Materials
Energetic Mono-, Di-, and Trisubstituted Nitroiminotetrazoles†
- Pages: 564-567
- First Published: 29 December 2008
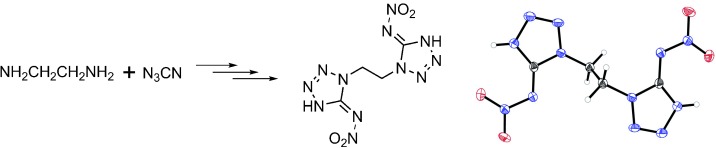
A bundle of energy: The title compounds were synthesized in good yield from aminotetrazoles (obtained from the reaction of cyanogen azide with primary amines) by treatment with 100 % nitric acid and were fully characterized by spectroscopic methods, elemental analysis, and in some cases X-ray diffraction (see example; N blue, O red). The heats of formation of these energetic materials were calculated, as well as their detonation pressures and velocities.
Coordination Modes
Planar Tetranuclear and Dumbbell-Shaped Octanuclear Palladium Complexes with Bridging Silylene Ligands†
- Pages: 568-571
- First Published: 29 December 2008
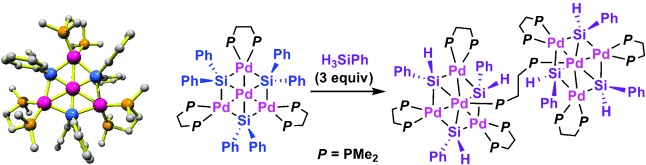
An uncommon structure: The novel title compounds (see scheme) were prepared and fully characterized. The tetranuclear complex contains a hexagonal Pd4Si3 core involving one central Pd atom, three outer Pd atoms, and three bridging Si atoms within the same plane, whereas the dumbbell-shaped octanuclear Pd complex is composed of two Pd4Si3 groups bridged by a diphosphine ligand.
Domino Reactions
Palladium-Catalyzed Annulation of Acyloximes with Arynes (or Alkynes): Synthesis of Phenanthridines and Isoquinolines†
- Pages: 572-577
- First Published: 29 December 2008
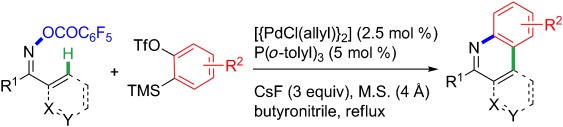
Intermolecular insertion: A palladium-catalyzed domino aminopalladation/ CH functionalization sequence has been developed, and provides access to functionalized phenanthridines and isoquinolines (see scheme; Tf=triflate, TMS=trimethylsilyl, M.S.=molecular sieves). The use of butyronitrile as the solvent is determinant to the success of the domino process.
Natural Product Synthesis
Total Synthesis of Bafilomycin A1†
- Pages: 578-581
- First Published: 29 December 2008
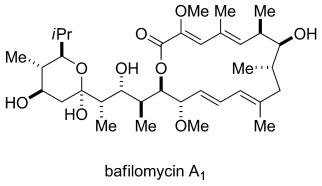
A convergent synthesis of bafilomycin A1 (see structure) is presented, and relies on the Zn(OTf)2-mediated diastereoselective addition of alkynes to aldehydes. The coupling of a complex enyne with a sensitive aldehyde in the key step, in combination with a novel strategy for a chemoselective trans-reduction of the enyne, establishes an alternative to standard palladium-catalyzed cross-coupling strategies for the formation of 1,3-dienes.
Polyhydride Clusters
Tetrameric Iridium Hydride-Rich Clusters Formed under Hydrogenation Conditions†
- Pages: 582-585
- First Published: 29 December 2008
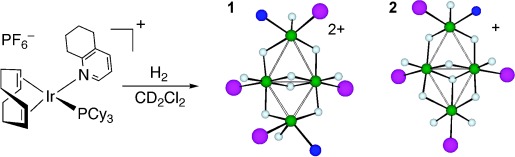
Butterfly clusters: Hydrogenation of a cationic iridium complex with the sterically bulky N-ligand tetrahydroquinoline (thq) leads to the formation of two distinct tetrameric polyhydrides (see scheme; Ir green, N blue, P purple), which differ by exchanging one thq ligand for hydride. Both complexes have butterfly structures, with similar core geometries, but markedly different hinge angles (40.8° for 1 and 9.1° for 2).
Radiochemistry
Fluorous Synthesis of 18F Radiotracers with the [18F]Fluoride Ion: Nucleophilic Fluorination as the Detagging Process†
- Pages: 586-589
- First Published: 29 December 2008
![Fluorous Synthesis of 18F Radiotracers with the [18F]Fluoride Ion: Nucleophilic Fluorination as the Detagging Process](/cms/asset/f2d5f85e-800d-4c4d-8530-0e6ec0a9ee8d/mcontent.jpg)
Tag team: The fluoro-detagging of fluorous sulfonates by the [18F]fluoride ion was found to be an advantageous strategy for the preparation of various 18F-labeled prosthetic groups and known radiotracers (see picture). Fluorous solid phase extraction (FSPE) was used to separate the excess fluorous precursor from the labeled material, which suggests that traditional purification protocols such as distillation or tedious separation can be avoided.
Asymmetric Amplification
Generation of Highly Enantioenriched Crystalline Products in Reversible Asymmetric Reactions with Racemic or Achiral Catalysts†
- Pages: 590-594
- First Published: 29 December 2008
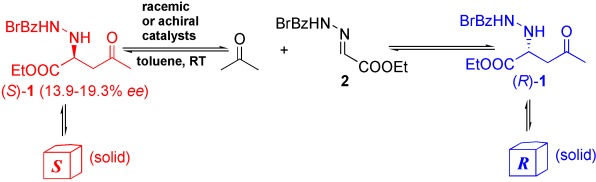
Backwards is better: By combining forward and backward reaction steps of a reversible chemical process (see scheme) with the physical processes of crystallization, crystal crushing, and dissolution, asymmetric amplification up to 100 % ee can be achieved in the presence of racemic or achiral catalysts starting from a conglomerate of the reaction product 1 with a low enantiomeric excess.
Protein–Inhibitor Complexes
The Absolute Configuration of Rhizopodin and Its Inhibition of Actin Polymerization by Dimerization†
- Pages: 595-598
- First Published: 29 December 2008
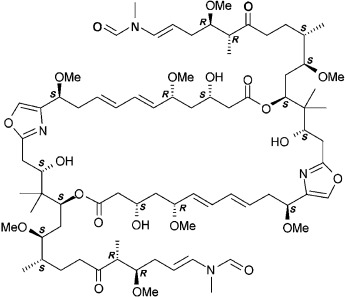
Three's company: Rhizopodin is a cytostatic macrolide and a potent actin depolymerizer produced by the myxobacterium Myxococcus stipitatus. A crystal structure analysis of the rhizopodin/actin complex reveals that rhizopodin is a C2-symmetric bislactone (see formula). The ternary complex supports the mode of rhizopodin-induced actin dimerization and reveals the absolute configuration and biologically active conformation of this macrolide.
Black Porphyrins
“Blackening” Porphyrins by Conjugation with Quinones†
- Pages: 599-603
- First Published: 29 December 2008
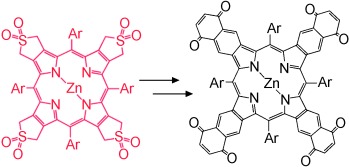
From red to black porphyrins: “Black” porphyrins absorb visible light effectively at all wavelengths and are available by conjugation of four benzoquinone units to a porphyrin core. These robust molecular components are likely to be useful in optoelectronic devices as they absorb a large fraction of the visible light.
Platinum Catalysis
A Highly Strained Planar-Chiral Platinacycle for Catalytic Activation of Internal Olefins in the Friedel–Crafts Alkylation of Indoles†
- Pages: 604-606
- First Published: 29 December 2008
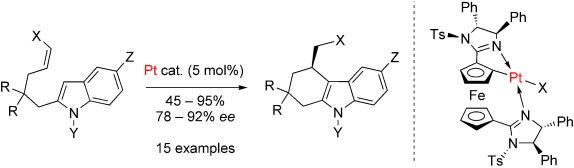
Activation by deformation: A planar-chiral platinacycle readily prepared by diastereoselective cycloplatination enables the enantioselective intramolecular hydroarylation of indoles having disubstituted Z olefins (see scheme; Ts=p-tolylsulfonyl). Sufficient activity is achieved by a combination of highly strained catalyst geometry and accelerated olefin coordination. This application represents the first highly enantioselective reaction catalyzed by a platinacycle.
Iron Catalysis
Domino Iron Catalysis: Direct Aryl–Alkyl Cross-Coupling†
- Pages: 607-610
- First Published: 29 December 2008
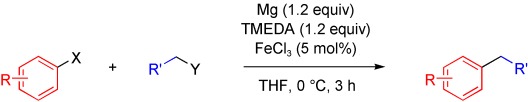
Striking while the iron is hot: Cheap FeCl3 serves as the precatalyst for the direct cross-coupling of aryl and alkyl halides that is based on the sequence of Grignard formation and subsequent cross-coupling. This one-pot reaction obviates preformation of hazardous Grignard compounds and limits the amount of reactive organomagnesium intermediates to low quasi-stationary concentrations. TMEDA=N,N,N′,N′-tetramethylethylenediamine.
Biocatalysis
Monitoring Catalysis of the Membrane-Bound Hydrogenase from Ralstonia eutropha H16 by Surface-Enhanced IR Absorption Spectroscopy †
- Pages: 611-613
- First Published: 29 December 2008
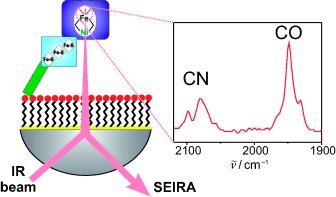
Immobilized biocatalyst: The oxygen-tolerant, membrane-bound hydrogenase of Ralstonia eutropha H16 is immobilized by a His-tag (see picture; green) onto a gold surface (yellow) modified with nickel–nitrilotriacetic acid (red/black). Catalytic activity towards hydrogen is investigated by surface-enhanced infrared absorption (SEIRA) spectroscopy. Switching redox states and related structural changes of the Ni–Fe active site are followed by the CO and CN− ligand stretching modes.