Tetrameric Iridium Hydride-Rich Clusters Formed under Hydrogenation Conditions†
Yingjian Xu Dr.
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorMehmet A. Celik
Istanbul Technical University, Faculty of Arts & Science (Turkey)
Search for more papers by this authorAmber L. Thompson Dr.
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorHairong Cai
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorMine Yurtsever Prof.
Istanbul Technical University, Faculty of Arts & Science (Turkey)
Search for more papers by this authorBarbara Odell Dr.
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorJennifer C. Green Prof.
Inorganic Chemistry Laboratory, Oxford University (UK)
Search for more papers by this authorD. Michael P. Mingos Prof.
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorJohn M. Brown Dr.
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorYingjian Xu Dr.
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorMehmet A. Celik
Istanbul Technical University, Faculty of Arts & Science (Turkey)
Search for more papers by this authorAmber L. Thompson Dr.
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorHairong Cai
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorMine Yurtsever Prof.
Istanbul Technical University, Faculty of Arts & Science (Turkey)
Search for more papers by this authorBarbara Odell Dr.
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorJennifer C. Green Prof.
Inorganic Chemistry Laboratory, Oxford University (UK)
Search for more papers by this authorD. Michael P. Mingos Prof.
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorJohn M. Brown Dr.
Chemistry Research Laboratory, Oxford University, 12 Mansfield Rd., Oxford OX1 3TA (UK), Fax: (+44) 1865-285-002 http://www.chem.ox.ac.uk/researchguide/jbrown.html
Search for more papers by this authorWe thank Merck for an unrestricted grant (Y.X.) and Oxford and Istanbul Technical Universities (ITU-HPCC) for computing facilities. TÜBİTAK provided a six-month scholarship that enabled M.A.C. to work in Oxford in 2007. We gratefully acknowledge help from Dr. T. D. W. Claridge (NMR); Dr. N. Oldham and Dr. J. McCullagh (MS). Johnson-Matthey kindly provided a loan of iridium salts. Prof. Andrew Weller provided very helpful comments on this manuscript.
Graphical Abstract
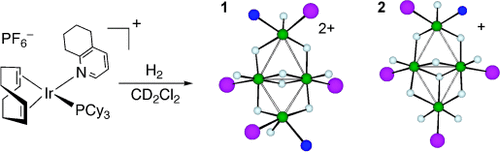
Butterfly clusters: Hydrogenation of a cationic iridium complex with the sterically bulky N-ligand tetrahydroquinoline (thq) leads to the formation of two distinct tetrameric polyhydrides (see scheme; Ir green, N blue, P purple), which differ by exchanging one thq ligand for hydride. Both complexes have butterfly structures, with similar core geometries, but markedly different hinge angles (40.8° for 1 and 9.1° for 2).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200804484_sm_miscellaneous_information.pdf885.5 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Reviews:
- 1aS. J. Roseblade, A. Pfaltz, Acc. Chem. Res. 2007, 40, 1402–1411;
- 1bS. J. Roseblade, A. Pfaltz, C. R. Chim. 2007, 10, 178–187;
- 1cT. L. Church, P. G. Andersson, Coord. Chem. Rev. 2008, 252, 513–531.
- 2Y. Xu, D. M. P. Mingos, J. M. Brown, Chem. Commun. 2008, 199–201.
- 3
- 3aD. F. Chodosh, R. H. Crabtree, H. Felkin, S. Morehouse, G. E. Morris, Inorg. Chem. 1982, 21, 1307–1311;
- 3bH. H. Wang, L. H. Pignolet, Inorg. Chem. 1980, 19, 1470–1480;
- 3cA. L. Casalnuovo, L. H. Pignolet, J. W. A. van der Velden, J. J. Bour, J. J. Steggerda, J. Am. Chem. Soc. 1983, 105, 5957–5958;
- 3dH. H. Wang, A. L. Casalnuovo, B. J. Johnson, A. M. Mueting, L. H. Pignolet, Inorg. Chem. 1988, 27, 325–331;
- 3eS. P. Smidt, A. Pfaltz, E. Martinez-Viviente, P. S. Pregosin, A. Albinati, Organometallics 2003, 22, 1000–1009.
- 4
- 4aA. S. Weller, J. S. McIndoe, Eur. J. Inorg. Chem. 2007, 4411–4423;
- 4bP. J. Dyson, J. S. McIndoe, Angew. Chem. 2004, 116, 6152–6154;
10.1002/ange.200460924 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 6028–6030.
- 5CCDC 692069 and 692070 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. Crystallographic data: 3 a: C90.75H165.50Cl1.50F12Ir4N2P6; Mr=2493.48; crystal size 0.04×0.11×0.13 mm; monoclinic (C2); a=22.8325(3), b=17.2007(3), c=16.6365(3) Å; β=118.473(1)°; V=5743.42(16) Å3; Z=2; μ=4.794 mm−1; ρcalcd=1.457 g cm−3; T=150(2) K; 45 368 reflections measured; 1296 independent reflections (Rint=0.1119); R1=0.0708; wR2=0.1730 [I>2σ(I)]; Δρmin=−2.841, Δρmax=5.995 e Å3. 3 b: C82H160.5Cl2F6Ir4N1O3.25P5; Mr=2316.83; crystal size 0.08×0.12×0.14 mm; triclinic (P
 ); a=14.59410(10), b=17.6600(2), c=20.9926(2) Å; α=108.3094(5), β=103.8296(4), γ=93.7822(5)°; V=4928.91(8) Å3; Z=2; μ=5.570 mm−1, ρcalcd=1.553 g cm−3; T=150(2) K; 37 501 reflections measured; 22 323 independent reflections (Rint=0.115); R1=0.0557; wR2=0.1450 [I>2σ(I)]; Δρmin=−3.69, Δρmax=4.09 e Å3.
); a=14.59410(10), b=17.6600(2), c=20.9926(2) Å; α=108.3094(5), β=103.8296(4), γ=93.7822(5)°; V=4928.91(8) Å3; Z=2; μ=5.570 mm−1, ρcalcd=1.553 g cm−3; T=150(2) K; 37 501 reflections measured; 22 323 independent reflections (Rint=0.115); R1=0.0557; wR2=0.1450 [I>2σ(I)]; Δρmin=−3.69, Δρmax=4.09 e Å3.
- 6aGeometry optimizations of the Ir tetramers were carried out by density functional theory (DFT) methods with the empirically parameterized hybrid functional B3LYP as implemented in Gaussian 03. The equilibrium structures have been characterized by a lack of imaginary vibrations. The 6-31g(d) basis set was applied to all elements except Ir which is described with the LANL2DZ basis set;
- 6bNMR spectroscopic calculations were carried out using the ADF 2007.01 program suite: ADF2007.01, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, http://www.scm.com. Further details are given in the Supporting Information.
- 7H. Hu, K. Krishnamurthy, J. Magn. Reson. 2006, 182, 173–177; MacKinetics (Leipold Associates) was used for the data simulation in Figure 5.
- 8D. M. P. Mingos, A. S. May in The Chemistry of Metal Cluster Complexes (Ed.: ), VCH, Weinheim, 1990, pp. 11–119.
- 9For a tetrahedral tetrameric hydride, [Ir4H8(CO)4(PPh3)4], see: L. Garlaschelli, F. Greco, G. Peli, M. Manassero, M. Sansoni, R. Gobetto, L. Salassa, R. D. Pergola, Eur. J. Inorg. Chem. 2003, 2108–2112.
- 10
- 10aFor a butterfly Pt4 cationic heptahydride tetramer see: R. J. Goodfellow, E. M. Hamon, J. A. K. Howard, J. L. Spencer, D. G. Turner, J. Chem. Soc. Chem. Commun. 1984, 1604–1606;
- 10bfor a planar rhomboidal Re2Au2 tetrameric hexahydride see: B. R. Sutherland, D. M. Ho, J. C. Huffman, K. G. Caulton, Angew. Chem. 1987, 99, 147–149; Angew. Chem. Int. Ed. Engl. 1987, 26, 135–137.
- 11V. Stevanovic, Z. Sljivancanin, A. Baldereschi, Phys. Rev. Lett. 2007, 99, 165501–165504.
- 12
- 12aM. J. Ingleson, M. F. Mahon, P. R. Raithby, A. S. Weller, J. Am. Chem. Soc. 2004, 126, 4784–4785;
- 12bS. K. Brayshaw, M. J. Ingleson, J. C. Green, J. S. McIndoe, P. R. Raithby, G. Kociok-Koehn, A. S. Weller, J. Am. Chem. Soc. 2006, 128, 6247–6263;
- 12cS. K. Brayshaw, J. C. Green, R. Edge, E. J. L. McInnes, P. R. Raithby, J. E. Warren, A. S. Weller, Angew. Chem. 2007, 119, 7990–7994;
10.1002/ange.200703069 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 7844–7848, and intervening references.
- 13A combination of NMR spectroscopy and DFT calculations was also used to define the hydride positions of unsymmetrical cationic [Rh6P6Hn] (n=12,14, 16) clusters: S. K. Brayshaw, J. C. Green, N. Hazari, A. S. Weller, Dalton Trans. 2007, 1781–1792.
- 14R. D. Adams, B. Captain, M. D. Smith, Angew. Chem. 2006, 118, 1127–1130;
10.1002/ange.200503736 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 1109–1112; R. D. Adams, B. Captain, L. Zhu, J. Am. Chem. Soc. 2007, 129, 2454–2455.