A Simple Preparation of Pyridine-Derived N-Heterocyclic Carbenes and Their Transformation into Bridging Ligands by Orthometalation†
Javier A. Cabeza Prof.
Departamento de Química Orgánica e Inorgánica-IUQOEM, Universidad de Oviedo-CSIC, 33071 Oviedo (Spain), Fax: (+34) 98510-3446
Search for more papers by this authorIgnacio del Río Prof.
Departamento de Química Orgánica e Inorgánica-IUQOEM, Universidad de Oviedo-CSIC, 33071 Oviedo (Spain), Fax: (+34) 98510-3446
Search for more papers by this authorEnrique Pérez-Carreño Prof.
Departamento de Química Física y Analítica Universidad de Oviedo, 33071 Oviedo (Spain)
Search for more papers by this authorM. Gabriela Sánchez-Vega Dr.
Departamento de Química Orgánica e Inorgánica-IUQOEM, Universidad de Oviedo-CSIC, 33071 Oviedo (Spain), Fax: (+34) 98510-3446
Search for more papers by this authorDigna Vázquez-García Dr.
Departamento de Química Orgánica e Inorgánica-IUQOEM, Universidad de Oviedo-CSIC, 33071 Oviedo (Spain), Fax: (+34) 98510-3446
Search for more papers by this authorJavier A. Cabeza Prof.
Departamento de Química Orgánica e Inorgánica-IUQOEM, Universidad de Oviedo-CSIC, 33071 Oviedo (Spain), Fax: (+34) 98510-3446
Search for more papers by this authorIgnacio del Río Prof.
Departamento de Química Orgánica e Inorgánica-IUQOEM, Universidad de Oviedo-CSIC, 33071 Oviedo (Spain), Fax: (+34) 98510-3446
Search for more papers by this authorEnrique Pérez-Carreño Prof.
Departamento de Química Física y Analítica Universidad de Oviedo, 33071 Oviedo (Spain)
Search for more papers by this authorM. Gabriela Sánchez-Vega Dr.
Departamento de Química Orgánica e Inorgánica-IUQOEM, Universidad de Oviedo-CSIC, 33071 Oviedo (Spain), Fax: (+34) 98510-3446
Search for more papers by this authorDigna Vázquez-García Dr.
Departamento de Química Orgánica e Inorgánica-IUQOEM, Universidad de Oviedo-CSIC, 33071 Oviedo (Spain), Fax: (+34) 98510-3446
Search for more papers by this authorThis work has been supported by the Spanish Ministerio de Ciencia e Innovación (projects CTQ2007-60865 and MAT2006-1997). Fellowships from the University of Carabobo (Venezuela, to M.G.S.-V.) and Xunta de Galicia (Spain, to D.V.-G.) are also acknowledged.
Graphical Abstract
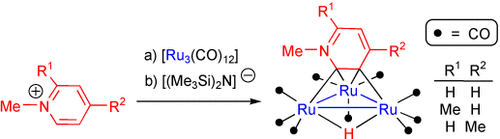
Pyrid-2-ylidenes are trapped in solution by [Ru3(CO)12] after being formed by deprotonation of N-substituted pyridinium cations. The great basicity of these NHC ligands and the polynuclear character of the ruthenium cluster trigger room temperature orthometalation of the initial κ1-C2-pyrid-2-ylidene ligands, leading to unprecedented face-capping κ2-C2,C3-pyrid-3-yl-2-ylidene ligands.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200804945_sm_miscellaneous_information.pdf283.5 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent general reviews, see:
- 1aF. E. Hahn, M. C. Jahnke, Angew. Chem. 2008, 120, 3166–3216; Angew. Chem. Int. Ed. 2008, 47, 3122–3172;
- 1bH. Clavier, K. Grela, A. Kirschning, M. Mauduit, S. P. Nolan, Angew. Chem. 2007, 119, 6906–6922;
10.1002/ange.200605099 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6786–6801.
- 2For recent reviews, see:
- 2aF. A. Glorius, Top. Organomet. Chem. 2007, 21, 1–20;
- 2b N-Heterocyclic Carbenes in Synthesis (Ed.: ), Wiley-VCH, Weinheim, 2006;
- 2cF. E. Hahn, Angew. Chem. 2006, 118, 1374–1378; Angew. Chem. Int. Ed. 2006, 45, 1348–1352.
- 3For a review of one-N, six-membered, heterocyclic carbene complexes, see: H. G. Raubenheimer, S. Cronje, Dalton Trans. 2008, 1265–1272.
- 4aC. P. Newman, G. J. Clarkson, N. W. Alcock, J. P. Rourke, Dalton Trans. 2006, 3321–3325;
- 4bW. H. Meyer, M. Deetlefs, M. Pohlmann, R. Scholz, M. W. Esterhuysen, G. R. Julius, H. G. Raubenheimer, Dalton Trans. 2004, 413–420;
- 4cG. W. V. Cave, A. J. Hallett, W. Errington, J. P. Rourke, Angew. Chem. 1998, 110, 3466–3468;
10.1002/(SICI)1521-3757(19981204)110:23<3466::AID-ANGE3466>3.0.CO;2-S Google ScholarAngew. Chem. Int. Ed. 1998, 37, 3270–3272;
- 4dH. G. Raubenheimer, J. G. Toerien, G. J. Kruger, R. Otte, W. van Zyl, P. Olivier, J. Organomet. Chem. 1994, 466, 291–295;
- 4eR. Aumann, P. Hinterding, Chem. Ber. 1992, 125, 2765–2772;
- 4fB. Crociani, F. Di Bianca, A. Giovenco, A. Scrivanti, J. Organomet. Chem. 1983, 251, 393–411;
- 4gP. J. Fraser, W. R. Roper, F. G. A. Stone, J. Chem. Soc. Dalton Trans. 1974, 760–764.
- 5
- 5aM. Albrecht, H. Stoeckli-Evans, Chem. Commun. 2005, 4705–4707;
- 5bS. K. Schneider, P. Roembke, G. R. Julius, C. Loschen, H. G. Raubenheimer, G. Frenking, W. A. Herrmann, Eur. J. Inorg. Chem. 2005, 2973–2977.
- 6J. S. Owen, J. A. Labinger, J. E. Bercaw, J. Am. Chem. Soc. 2004, 126, 8247–8255.
- 7F. P. Fanizzi, G. J. Sunley, J. A. Wheeler, H. Adams, N. A. Bailey, P. M. Maitlis, Organometallics 1990, 9, 131–136.
- 8S. Gómez-Bujedo, M. Alcarazo, C. Pichon, E. Alvarez, R. Fernández, J. M. Lassaletta, Chem. Commun. 2007, 1180–1182.
- 9S. H. Wiedemann, J. C. Lewis, J. A. Ellman, R. G. Bergman, J. Am. Chem. Soc. 2006, 128, 2452–2462.
- 10
- 10aS. Conejero, P. Lara, M. Paneque, A. Petronilho, M. L. Poveda, O. Serrano, F. Batiré, E. Alvarez, C. Moya, V. Salazar, E. Carmona, Angew. Chem. 2008, 120, 4452–4455;
10.1002/ange.200800705 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4380–4383;
- 10bE. Alvarez, S. Conejero, P. Lara, J. A. López, M. Paneque, A. Petronilho, M. L. Poveda, D. del Río, O. Serrano, E. Carmona, J. Am. Chem. Soc. 2007, 129, 14130–14131;
- 10cE. Alvarez, S. Conejero, M. Paneque, A. Petronilho, M. L. Poveda, O. Serrano, E. Carmona, J. Am. Chem. Soc. 2006, 128, 13060–13061.
- 11
- 11aM. L. Buil, M. A. Esteruelas, K. Garcés, M. Oliván, E. Oñate, J. Am. Chem. Soc. 2007, 129, 10998–10999;
- 11bM. A. Esteruelas, F. J. Fernández-Alvarez, E. Oñate, Organometallics 2007, 26, 5239–5245;
- 11cM. A. Esteruelas, F. J. Fernández-Alvarez, E. Oñate, J. Am. Chem. Soc. 2006, 128, 13044–13045.
- 12G. Song, Y. Li, S. Chen, X. Li, Chem. Commun. 2008, 3558–3560.
- 13A toluene solution of K[(Me3Si)2N] (315 μL, 0.5 M, 0.156 mmol) was added to a mixture of [Ru3(CO)12] (100 mg, 0.156 mmol) and N-methylpyridinium triflate (38 mg, 0.156 mmol) in THF (20 mL). The mixture was stirred at room temperature until the consumption of the starting ruthenium complex was confirmed by IR spectroscopy (10 h). The solvent was removed under reduced pressure and the residue was extracted into toluene (20 mL) to give a red solution and a brown solid. The solution was concentrated and purified by preparative thin-layer chromatography (silica gel). Elution with dichloromethane/hexane (1:4) afforded compound 1 (10 mg, 10 %) as a red-orange band. A dark brown residue remained uneluted on the plate baseline. Compounds 2 and 3 were prepared in similar yields from the respective appropriate N-methylpicolinium triflate.
- 14Spectroscopic data:
- 14a1: 1H NMR (CDCl3): δ=8.28 (d, J=6.5 Hz, 1 H), 7.99 (d, J=6.5 Hz, 1 H), 6.82 (t, J=6.5 Hz, 1 H), 4.09 (s, 3 H), −18.08 ppm (s, 1 H); IR (CH2Cl2):
 (CO)=2079 (m), 2052 (s), 2013 (vs), 1981 (m, sh), 1950 cm−1 (w, sh);
(CO)=2079 (m), 2052 (s), 2013 (vs), 1981 (m, sh), 1950 cm−1 (w, sh);
- 14b2: 1H NMR (CDCl3): δ=8.18 (d, J=7.5 Hz, 1 H), 6.69 (d, J=7.5 Hz, 1 H), 4.07 (s, 3 H), 3.68 (s, 3 H), −17.95 ppm (s, 1 H); IR (CH2Cl2):
 (CO)=2078 (m), 2049 (s), 2013 (vs), 1981 (m, sh), 1949 cm−1 (w, sh);
(CO)=2078 (m), 2049 (s), 2013 (vs), 1981 (m, sh), 1949 cm−1 (w, sh);
- 14c3: 1H NMR (CDCl3): δ=7.95 (d, J=6.0 Hz, 1 H), 6.84 (d, J=6.0 Hz, 1 H), 4.05 (s, 3 H), 2.54 (s, 3 H), −17.76 ppm (s, 1 H); 13C NMR (DEPT, CDCl3): δ=177.7 (C), 174.5 (C), 146.5 (C), 143.1 (CH), 115.8 (CH), 50.7 (CH3), 26.8 ppm (CH3); IR (CH2Cl2):
 (CO)=2077 (m), 2048 (s), 2013 (vs), 1986 (m, sh), 1945 cm−1 (w, sh).
(CO)=2077 (m), 2048 (s), 2013 (vs), 1986 (m, sh), 1945 cm−1 (w, sh).
- 15Crystal data for 3⋅(hexane)0.25: C16H9NO9Ru3⋅0.25(C6H14); Mr=680.46; crystal size 0.12×0.07×0.02 mm; triclinic; space group P
 ; a=9.630(2), b=9.910(2), c=12.358(3) Å; α=80.472(1), β=71.785(1), γ=79.735(1)°; V=1094.7(4) Å3; Z=2; ρcalcd=2.064 g cm−3; F(000)=650; μ=2.090 mm−1; 10 691 reflections measured, 3640 independent reflections; R1=0.0609 and wR2=0.1996 (all data). CCDC 703646 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
; a=9.630(2), b=9.910(2), c=12.358(3) Å; α=80.472(1), β=71.785(1), γ=79.735(1)°; V=1094.7(4) Å3; Z=2; ρcalcd=2.064 g cm−3; F(000)=650; μ=2.090 mm−1; 10 691 reflections measured, 3640 independent reflections; R1=0.0609 and wR2=0.1996 (all data). CCDC 703646 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 16
- 16aM. R. Crittall, C. E. Ellul, M. F. Mahon, O. Saker, M. K. Whittlesey, Dalton Trans. 2008, 4209–4211;
- 16bC. E. Ellul, M. F. Mahon, O. Saker, M. K. Whittlesey, Angew. Chem. 2007, 119, 6459–6461;
10.1002/ange.200701930 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 6343–6345;
- 16cC. E. Ellul, O. Saker, M. F. Mahon, D. C. Apperley M. K. Whittlesey, Organometallics 2008, 27, 100–108.
- 17
- 17aJ. A. Cabeza, E. Pérez-Carreño, Organometallics 2008, 27, 4697–4702;
- 17bJ. A. Cabeza, I. del Río, D. Miguel, E. Pérez-Carreño, M. G. Sánchez-Vega, Organometallics 2008, 27, 211–217;
- 17cJ. A. Cabeza, I. del Río, D. Miguel, E. Pérez-Carreño, M. G. Sánchez-Vega, Dalton Trans. 2008, 1937–1942;
- 17dJ. A. Cabeza, I. del Río, D. Miguel, M. G. Sánchez-Vega, Angew. Chem. 2008, 120, 1946–1948;
10.1002/ange.200705154 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 1920–1922;
- 17eJ. A. Cabeza, I. del Río, D. Miguel, M. G. Sánchez-Vega, Chem. Commun. 2005, 3956–3958.
- 18M. I. Bruce, M. L. Cole, R. S. C. Fung, C. M. Forsyth, M. Hilder, P. C. Junk, K. Konstas, Dalton Trans. 2008, 4118–4128.
- 19The experimental details of the DFT calculations, as well as tables containing computed interatomic distances, NPA atomic charges, and Cartesian atomic coordinates for all optimized stationary points are contained in the Supporting Information.