Helical Poly(quinoxaline-2,3-diyl)s Bearing Metal-Binding Sites as Polymer-Based Chiral Ligands for Asymmetric Catalysis†
Takeshi Yamamoto
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2722 http://www.sbchem.kyoto-u.ac.jp/suginome-lab/
Search for more papers by this authorMichinori Suginome Prof. Dr.
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2722 http://www.sbchem.kyoto-u.ac.jp/suginome-lab/
Search for more papers by this authorTakeshi Yamamoto
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2722 http://www.sbchem.kyoto-u.ac.jp/suginome-lab/
Search for more papers by this authorMichinori Suginome Prof. Dr.
Department of Synthetic Chemistry and Biological Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510 (Japan), Fax: (+81) 75-383-2722 http://www.sbchem.kyoto-u.ac.jp/suginome-lab/
Search for more papers by this authorThis work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology (Japan) and the Ogasawara Foundation for the Promotion of Science and Engineering.
Graphical Abstract
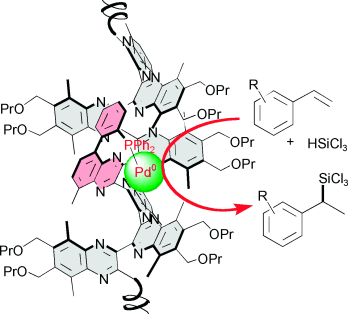
Living it up: Helical polyquinoxalines with single and multiple metal-binding sites, prepared by living polymerization of o-diisocyanobenzenes, are used in the asymmetric hydrosilylation of styrenes, resulting in comparable enantioselectivities to those obtained by low-molecular-weight catalyst systems (up to 87 % ee, stereochemistry was determined by a chiral initiator) and a turnover number of almost 1000.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200803719_sm_miscellaneous_information.pdf3.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Nakano, Y. Okamoto, Chem. Rev. 2001, 101, 4013;
- 1bJ. J. L. Cornelissen, A. E. Rowan, R. J. M. Nolte, N. A. J. M. Sommerdijk, Chem. Rev. 2001, 101, 4039;
- 1cE. Yashima, K. Maeda, T. Nishimura, Chem. Eur. J. 2004, 10, 42.
- 2Y. Okamoto, K. Suzuki, K. Ohta, K. Harada, H. Yuki, J. Am. Chem. Soc. 1979, 101, 4763.
- 3
- 3aY. Okamoto, M. Matsuda, T. Nakano, E. Yashima, Polym. J. 1993, 25, 391;
- 3bM. M. Green, N. C. Peterson, T. Sato, A. Teramoto, R. Cook, S. Lifson, Science 1995, 268, 1860.
- 4
- 4aP. C. J. Kamer, R. J. M. Nolte, W. Drenth, J. Am. Chem. Soc. 1988, 110, 6818;
- 4bT. J. Deming, B. M. Novak, J. Am. Chem. Soc. 1992, 114, 7926;
- 4cM. Ishikawa, K. Maeda, Y. Mitsutsuji, E. Yashima, J. Am. Chem. Soc. 2004, 126, 732;
- 4dR. J. M. Nolte, Chem. Soc. Rev. 1994, 23, 11;
- 4eA review on the polymerization of isocyanide: M. Suginome, Y. Ito, Adv. Polym. Sci. 2004, 171, 77.
- 5
- 5aF. Ciardelli, S. Lanzillo, O. Pieroni, Macromolecules 1974, 7, 174;
- 5bL. S. Moore, C. B. Gorman, R. H. Grubbs, J. Am. Chem. Soc. 1991, 113, 1704;
- 5cT. Aoki, T. Kaneko, N. Maruyama, A. Sumi, M. Takahashi, T. Sato, M. Teraguchi, J. Am. Chem. Soc. 2003, 125, 6346;
- 5dK. Maeda, H. Mochizuki, M. Watanabe, E. Yashima, J. Am. Chem. Soc. 2006, 128, 7639.
- 6
- 6aD. S. Schlitzer, B. M. Novak, J. Am. Chem. Soc. 1998, 120, 2196;
- 6bH.-Z. Tang, Y. Lu, G. Tian, M. D. Capracotta, B. M. Novak, J. Am. Chem. Soc. 2004, 126, 3722;
- 6cG. Tian, Y. Lu, B. M. Novak, J. Am. Chem. Soc. 2004, 126, 4082.
- 7Biopolymer-based chiral ligands: Polypeptide:
- 7aS. R. Gilbertson, X. Wang, Tetrahedron Lett. 1996, 37, 6475; DNA:
- 7bG. Roelfes, B. L. Feringa, Angew. Chem. 2005, 117, 3294;
10.1002/ange.200500298 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3230;
- 7cA. J. Boersma, B. L. Feringa, G. Roelfes, Org. Lett. 2007, 9, 3647;
- 7dD. Coquière, B. L. Feringa, G. Roelfes, Angew. Chem. 2007, 119, 9468;
10.1002/ange.200703459 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 9308.
- 8M. Reggelin, M. Schultz, M. Holbach, Angew. Chem. 2002, 114, 1684;
10.1002/1521-3757(20020503)114:9<1684::AID-ANGE1684>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1614.10.1002/1521-3773(20020503)41:9<1614::AID-ANIE1614>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 9C. Ren, C. Chen, F. Xi, T. Nakano, Y. Okamoto, J. Polym. Sci. Part A 1993, 31, 2721.
- 10
- 10aM. Reggelin, S. Doerr, M. Klussmann, M. Schultz, M. Holbach, Proc. Natl. Acad. Sci. USA 2004, 101, 5461;
- 10bF. Sanda, H. Araki, T. Masuda, Chem. Lett. 2005, 34, 1642;
- 10cC. A. Müller, T. Hoffart, M. Holbach, M. Reggelin, Macromolecules 2005, 38, 5375.
- 11L. Pu, Chem. Rev. 1998, 98, 2405.
- 12Y. Ito, E. Ihara, M. Murakami, M. Shiro, J. Am. Chem. Soc. 1990, 112, 6446.
- 13Y. Ito, T. Miyake, S. Hatano, R. Shima, T. Ohara, M. Suginome, J. Am. Chem. Soc. 1998, 120, 11880.
- 14
- 14aY. Ito, T. Ohara, R. Shima, M. Suginome, J. Am. Chem. Soc. 1996, 118, 9188;
- 14bY. Ito, T. Miyake, T. Ohara, M. Suginome, Macromolecules 1998, 31, 1697;
- 14cY. Ito, T. Miyake, M. Suginome, Macromolecules 2000, 33, 4034;
- 14dM. Suginome, S. Collet, Y. Ito, Org. Lett. 2002, 4, 351.
- 15The presence of a small excess of PMe2Ph in the polymerization reaction mixture was essential to obtain maximum screw-sense selectivity. In its absence, polymer P(10-1-10) was formed with lower screw-sense excess, leading to the enantioselectivity of 79 % ee in the hydrosilylation of styrene.
- 16H. Wu, J. Yu, J. B. Spencer, Org. Lett. 2004, 6, 4675.
- 17
- 17aY. Uozumi, T. Hayashi, J. Am. Chem. Soc. 1991, 113, 9887;
- 17bT. Hayashi, S. Hirate, K. Kitayama, H. Tsuji, A. Torii, Y. Uozumi, J. Org. Chem. 2001, 66, 1441;
- 17cT. Hayashi, Acc. Chem. Res. 2000, 33, 354.