Synthesis of [2]Catenanes by Oxidative Intramolecular Diyne Coupling Mediated by Macrocyclic Copper(I) Complexes†
Correction(s) for this article
-
Synthesis of [2]Catenanes by Oxidative Intramolecular Diyne Coupling Mediated by Macrocyclic Copper(I) Complexes
- Volume 48Issue 15Angewandte Chemie International Edition
- pages: 2630-2630
- First Published online: March 24, 2009
Yuta Sato
Department of Chemistry, Faculty of Science, Tokyo University of Science, Kagurazaka, Shinjuku, Tokyo 162-8601 (Japan) http://www.rs.kagu.tus.ac.jp/sslab/
Search for more papers by this authorRyu Yamasaki Dr.
Department of Chemistry, Faculty of Science, Tokyo University of Science, Kagurazaka, Shinjuku, Tokyo 162-8601 (Japan) http://www.rs.kagu.tus.ac.jp/sslab/
Search for more papers by this authorShinichi Saito Dr.
Department of Chemistry, Faculty of Science, Tokyo University of Science, Kagurazaka, Shinjuku, Tokyo 162-8601 (Japan) http://www.rs.kagu.tus.ac.jp/sslab/
Search for more papers by this authorYuta Sato
Department of Chemistry, Faculty of Science, Tokyo University of Science, Kagurazaka, Shinjuku, Tokyo 162-8601 (Japan) http://www.rs.kagu.tus.ac.jp/sslab/
Search for more papers by this authorRyu Yamasaki Dr.
Department of Chemistry, Faculty of Science, Tokyo University of Science, Kagurazaka, Shinjuku, Tokyo 162-8601 (Japan) http://www.rs.kagu.tus.ac.jp/sslab/
Search for more papers by this authorShinichi Saito Dr.
Department of Chemistry, Faculty of Science, Tokyo University of Science, Kagurazaka, Shinjuku, Tokyo 162-8601 (Japan) http://www.rs.kagu.tus.ac.jp/sslab/
Search for more papers by this authorWe thank the Yamada Science Foundation and the UBE Foundation for the financial support of this work.
Graphical Abstract
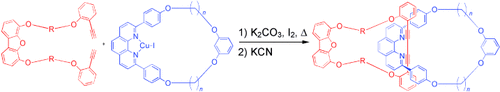
Right said thread: Oxidative intramolecular coupling reactions of α,ω-diynes in the presence of macrocyclic phenanthroline CuI complexes allows the synthesis of [2]catenanes in up to 64 % yield (see scheme). The bond-forming reaction leads to concurrent threading of the diyne through the phenanthroline macrocycle.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200804864_sm_miscellaneous_information.pdf4.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aV. Serreli, C. F. Lee, E. R. Key, D. A. Leigh, Nature 2007, 445, 523–527;
- 1bV. Balzani, A. Credi, B. Ferrer, S. Silvi, M. Venturi, Top. Curr. Chem. 2005, 262, 1–27;
- 1cC. P. Mandl, B. König, Angew. Chem. 2004, 116, 1650–1652;
10.1002/ange.200301697 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1622–1624;
- 1dJ. V. Hernandez, E. R. Key, D. A. Leigh, Science 2004, 306, 1532–1537.
- 2
- 2aS. Bonnet, J. P. Collin, M. Koizumi, P. Mobian, J. P. Sauvage, Adv. Mater. 2006, 18, 1239–1250;
- 2bJ. E. Green, J. W. Choi, A. Boukai, Y. Bunimovich, E. Johonston-Halperin, E. Delonno, Y. Luo, B. A. Sheri ff, K. Xu, Y. S. Shin, H. R. Tseng, J. F. Stoddart, J. R. Health, Nature 2007, 445, 414–417;
- 2cA. H. Flood, J. F. Stoddart, J. R. Health, Science 2004, 306, 2055–2056;
- 2d Molecular Switches (Eds.: ), Wiley-VCH, Weinheim, 2001.
- 3E. J. Wasserman, J. Am. Chem. Soc. 1960, 82, 4433–4434.
- 4
- 4aG. Schill, C. Zürcher, Angew. Chem. 1969, 81, 996–997;
10.1002/ange.19690812307 Google ScholarAngew. Chem. Int. Ed. Engl. 1969, 8, 988–988;
- 4bG. Schill, E. Logemam, W. Vetter, Angew. Chem. 1972, 84, 1144–1145;
10.1002/ange.19720842306 Google ScholarAngew. Chem. Int. Ed. Engl. 1972, 11, 1089–1090;
- 4cFor review, see, G. Schill in Catenanes, Rotaxanes, and Knots (Eds: ), Academic Press, New York, 1971.
- 5
- 5aC. O. Dietrich-Buchecker, J. P. Sauvage, J. P. Kintzinger, Tetrahedron Lett. 1983, 24, 5095–5098;
- 5bC. O. Dietrich-Buchecker, J. P. Sauvage, Chem. Rev. 1987, 87, 795–810.
- 6aF. M. Raymo, J. F. Stoddart, Chem. Rev. 1999, 99, 1643–1663;
- 6bT. Ikeda, S. Saha, I. Aprahamian, K. C. F. Leung, A. Williams, W. Q. Deng, A. H. Flood, W. A. Goddart, J. F. Stoddart, Chem. Asian J. 2007, 2, 76–93;
- 6cM. Fujita, F. Ibukuro, H. Hagihara, K. Ogura, Nature 1994, 367, 720–723.
- 7aC. A. Schalley, W. Reckien, S. Peyerimhoff, B. Baytekin, F. Vögtle, Chem. Eur. J. 2004, 10, 4777–4789;
- 7bF. Vögtle, S. Meier, R. Hoss, Angew. Chem. 1992, 104, 1628–1631;
10.1002/ange.19921041212 Google ScholarAngew. Chem. Int. Ed. Engl. 1992, 31, 1619;
- 7cA. M. L. Fuller, D. A. Leigh, P. J. Lusby, A. M. Z. Slawin, D. B. Walker, J. Am. Chem. Soc. 2005, 127, 12612–12619.
- 8J. Berná, J. D. Crowley, S. M. Goldup, K. D. Hänni, A. L. Lee, D. A. Leigh, Angew. Chem. 2007, 119, 5811–5815;
10.1002/ange.200701678 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 5709–5713.
- 9S. Saito, E. Takahashi, K. Nakazono, Org. Lett. 2006, 8, 5133–5136.
- 10V. Aucagne, K. D. Hänni, D. A. Leigh, P. J. Lusby, D. B. Walker, J. Am. Chem. Soc. 2006, 128, 2186–2187.
- 11V. Aucagne, J. Berná, J. D. Crowely, S. M. Goldup, K. D. Hänni, D. A. Leigh, P. J. Lusby, V. E. Ronaldson, A. M. Z. Slawin, A. Viterisi, D. B. Walker, J. Am. Chem. Soc. 2007, 129, 11950–11963.
- 12See the Supporting Information.
- 13At the same time, we examined the cyclization of 2 in the presence of an acyclic phenanthroline Cu complex. No correlation was evident between the yield of the cyclized product and the yield of the catenane.
- 14T. C. Higgs, S. Parsons, P. J. Bailey, A. C. Jones, F. McLachlan, A. Parkin, A. Dawson, P. A. Tasker, Organometallics 2002, 21, 5692–5702.
- 15The precise structure of the intermediate of this reaction is not clear, and CuI/CuIII interconversion could be another possibility. The final important intermediate, however, should be a monomeric dialkynyl Cu species. The dimeric Cu complex which is postulated in some reactions would not form as an intermediate for this reaction. See,
- 15aJ. Berná, S. M. Goldup, A. L. Lee, D. A. Leigh, M. D. Symes, G. Teobaldi, F. Zerbetto, Angew. Chem. 2008, 120, 4464–4468;
10.1002/ange.200800891 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4392–4396;
- 15bW. Y. Lo, C.-H. Lam, V. W. W. Yam, N. Zhu, K. K. Cheung, S. Fathallaf, S. Messaoudi, B. L. Guennic, S. Kahlal, J. F. Halet, J. Am. Chem. Soc. 2004, 126, 7300–7310;
- 15cP. Siemsen, R. C. Livingston, F. Diederich, Angew. Chem. 2000, 112, 2740–2767;
10.1002/1521-3757(20000804)112:15<2740::AID-ANGE2740>3.0.CO;2-F Google ScholarAngew. Chem. Int. Ed. 2000, 39, 2632–2657.10.1002/1521-3773(20000804)39:15<2632::AID-ANIE2632>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 16The macrocyclic Cu complexes used in this study are efficient catalysts for the oxidative coupling of small alkynes such as 4-methoxyphenylacetylene and the coupling product was isolated in more than 90 % yield in the presence of a catalytic amount (10 mol %) of the complex. Therefore, in principle, the reaction of a molecule of 1 would proceed with more than two molecules of the diyne, and a multicatenane could be isolated. However, we could not isolate a multicatenane from the reaction mixture. The study directed toward the formation of the multicatenane is ongoing.