Palladium-Catalyzed Annulation of Acyloximes with Arynes (or Alkynes): Synthesis of Phenanthridines and Isoquinolines†
Thibaud Gerfaud
Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France), Fax: (+33) 1-6907-7247 http://www.icsn.cnrs-gif.fr/article.php3?id_article=122
Search for more papers by this authorLuc Neuville Dr.
Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France), Fax: (+33) 1-6907-7247 http://www.icsn.cnrs-gif.fr/article.php3?id_article=122
Search for more papers by this authorJieping Zhu Dr.
Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France), Fax: (+33) 1-6907-7247 http://www.icsn.cnrs-gif.fr/article.php3?id_article=122
Search for more papers by this authorThibaud Gerfaud
Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France), Fax: (+33) 1-6907-7247 http://www.icsn.cnrs-gif.fr/article.php3?id_article=122
Search for more papers by this authorLuc Neuville Dr.
Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France), Fax: (+33) 1-6907-7247 http://www.icsn.cnrs-gif.fr/article.php3?id_article=122
Search for more papers by this authorJieping Zhu Dr.
Institut de Chimie des Substances Naturelles, CNRS, 91198 Gif-sur-Yvette Cedex (France), Fax: (+33) 1-6907-7247 http://www.icsn.cnrs-gif.fr/article.php3?id_article=122
Search for more papers by this authorFinancial support from CNRS and the Institut de Chimie des Substances Naturelles are gratefully acknowledged. T.G. thanks the institute for a doctoral fellowships.
Graphical Abstract
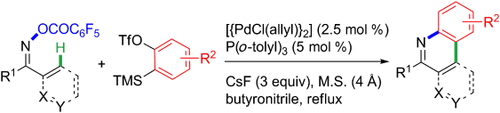
Intermolecular insertion: A palladium-catalyzed domino aminopalladation/ CH functionalization sequence has been developed, and provides access to functionalized phenanthridines and isoquinolines (see scheme; Tf=triflate, TMS=trimethylsilyl, M.S.=molecular sieves). The use of butyronitrile as the solvent is determinant to the success of the domino process.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200804683_sm_miscellaneous_information.pdf10.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aJ. F. Hartwig in Handbook of Organopalladium Chemistry for Organic Synthesis, Vol. 1 (Eds.: ), Wiley-Interscience, New York, 2002, pp. 1051–1096;
10.1002/0471212466.ch42 Google Scholar
- 1bA. R. Muci, S. L. Buchwald in Topics in Current Chemistry, Vol. 219 (Ed.: ), Springer, Berlin, 2002, pp. 133–205;
- 1cS. L. Buchwald, C. Mauger, G. Mignani, U. Scholz, Adv. Synth. Catal. 2006, 348, 23–39.
- 2
- 2aM. Kitamura, K. Narasaka, Chem. Rec. 2002, 2, 268–277;
- 2bK. Narasaka, M. Kitamura, Eur. J. Org. Chem. 2005, 4505–4519.
- 3Selected CN-containing coupling partner: benzophenone imine
- 3aJ. P. Wolfe, J. Khman, J. P. Sadighi, R. A. Singer, S. L. Buchwald, Tetrahedron Lett. 1997, 38, 6367–6370;
- 3bG. Mann, J. F. Hartwig, M. S. Driver, C. Fernandez-Rivas, J. Am. Chem. Soc. 1998, 120, 827–828; sulfoximines:
- 3cC. Bolm, J. P. Hildebrand, J. Org. Chem. 2000, 65, 169–175; N-trialkylsilylimines:
- 3dJ. Barluenga, F. Aznar, C. Valdés, Angew. Chem. 2004, 116, 347–349;
10.1002/ange.200352808 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 343–345.
- 4β CC bond cleavage from alkylideneaminopalladium(II) species have also been reported:
- 4aT. Nishimura, S. Uemura, J. Am. Chem. Soc. 2000, 122, 12049–12050;
- 4bT. Nishimura, Y. Nishiguchi, Y. Maeda, S. Uemura, J. Org. Chem. 2004, 69, 5342–5347.
- 5
- 5aH. Tsutsui, K. Narasaka, Chem. Lett. 1999, 45–56;
- 5bH. Tsutsui, K. Narasaka, M. Kitamura, Bull. Chem. Soc. Jpn. 2002, 75, 1451–1460;
- 5cJ. Ichikawa, K. Sakoda, J. Mihara, N. Ito, J. Fluorine Chem. 2006, 127, 489–504;
- 5dS. Zaman, M. Kitamura, A. D. Abell, Aust. J. Chem. 2007, 60, 624–626;
- 5eJ.-L. Zhu, Y.-H. Chan, Synlett 2008, 1250–1254.
- 6
- 6aH. Tsutsui, K. Narasaka, Chem. Lett. 2001, 526–527;
- 6bM. Kitamura, D. Kudo, K. Narasaka, ARKIVOC 2006, Part (iii), 148–162.
- 7
- 7aM. Kitamura, S. Zaman, K. Narasaka, Synlett 2001, 974–976;
- 7bM. Kitamura, S. Chiba, O. Saku, K. Narasaka, Chem. Lett. 2002, 606–607;
- 7cS. Zaman, M. Kitamura, K. Narasaka, Bull. Chem. Soc. Jpn. 2003, 76, 1055–1062;
- 7dS. Chiba, M. Kitamura, O. Saku, K. Narasaka, Bull. Chem. Soc. Jpn. 2004, 77, 785–796; imidazoles:
- 7eS. Zaman, K. Mitsuru, A. D. Abell, Org. Lett. 2005, 7, 609–611; isoxazolines:
- 7f P. J. Thomas, A. T. Axtell, J. Klosin, W. Peng, C. L. Rand, T. P. Clark, C. R. Landis, K. A. Abboud, Org. Lett. 2007, 9, 2665–2668.
- 8A. Fürstner, K. Radkowski, H. Peters, Angew. Chem. 2005, 117, 2837–2841;
10.1002/ange.200462215 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2777–2781.
- 9The copper-catalyzed reaction of acyloxime involving intermolecular transmetalation with boronic acid is known, see: S. Liu, L. S. Liebeskind, J. Am. Chem. Soc. 2008, 130, 6918–6919.
- 10
- 10aF. Bonnaterre, M. Bois-Choussy, J. Zhu, Org. Lett. 2006, 8, 4351–4354;
- 10bA. Salcedo, L. Neuville, J. Zhu, J. Org. Chem. 2008, 73, 3600–3603.
- 11
- 11aG. Cuny, M. Bois-Choussy, J. Zhu, Angew. Chem. 2003, 115, 4922–4925;
10.1002/ange.200351923 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4774–4777;
- 11bG. Cuny, M. Bois-Choussy, J. Zhu, J. Am. Chem. Soc. 2004, 126, 14475–14484;
- 11cA. Pinto, L. Neuville, P. Retailleau, J. Zhu, Org. Lett. 2006, 8, 4927–4930;
- 11dA. Pinto, L. Neuville, P. Retailleau, J. Zhu, Angew. Chem. 2007, 119, 3355–3359;
10.1002/ange.200605192 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3291–3295;
- 11eA. Pinto, Y. Jia, L. Neuville, J. Zhu, Chem. Eur. J. 2007, 13, 961–967;
- 11fA. Salcedo, L. Neuville, C. Rondot, P. Retailleau, J. Zhu, Org. Lett. 2008, 10, 857–860.
- 12Only limited examples of catalytic intermolecular aminopalladation of alkynes (alkenes) that involve amides, carbamates, and sulfonamides are known; for general reviews, see:
- 12aS. Cacchi, G. Fabrizi, Chem. Rev. 2005, 105, 2873–2920;
- 12bA. Minatti, K. Muñiz, Chem. Soc. Rev. 2007, 36, 1142–1152; for recent examples, see:
- 12cT. Shimada, Y. Yamamoto, J. Am. Chem. Soc. 2002, 124, 12670–12671;
- 12dS. Tang, P. Peng, S.-F. Pi, Y. Liang, N.-X. Wang, J.-H. Li, Org. Lett. 2008, 10, 1179–1182; for selected aminopalladation of alkenes and dienes, see:
- 12eT. Hosokawa, M. Takano, Y. Kuroki, S.-I. Murahashi, Tetrahedron Lett. 1992, 33, 6643–6646;
- 12fV. I. Timokhin, N. R. Anastasi, S. S. Stahl, J. Am. Chem. Soc. 2003, 125, 12996–12997;
- 12gG. L. J. Bar, G. C. Lloyd-Jones, K. I. Booker-Milburn, J. Am. Chem. Soc. 2005, 127, 7308–7309;
- 12hH. Du, B. Zhao, Y. Shi, J. Am. Chem. Soc. 2007, 129, 762–763; for a review on aza-Wacker reactions, see:
- 12iV. Kotov, C. C. Scarborough, S. S. Stahl, Inorg. Chem. 2007, 46, 1910–1923.
- 13For recent reviews dealing with CH activation, see:
- 13aL.-C. Campeau, K. Fagnou, Chem. Commun. 2006, 1253- 1264;
- 13bD. Alberico, M. E. Scott, M. Lautens, Chem. Rev. 2007, 107, 174–238; for a monograph, see: Handbook of CH Transformations (Ed.: ), Wiley-VCH, Weinheim, 2005.
- 14For reviews on arynes, see:
- 14aS. Saito, Y. Yamamoto, Chem. Rev. 2000, 100, 2901–2916;
- 14bH. H. Wenk, M. Winkler, W. Sander, Angew. Chem. 2003, 115, 518–546;
10.1002/ange.200390119 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 502–528;
- 14cH. Pellissier, M. Santelli, Tetrahedron 2003, 59, 701–730;
- 14dR. Sanz, Org. Prep. Proced. Int. 2008, 40, 215–291.
- 15
- 15aD. Peña, S. Escudero, D. Pérez, E. Guitián, L. Castedo, Angew. Chem. 1998, 110, 2804–2806;
10.1002/(SICI)1521-3757(19981002)110:19<2804::AID-ANGE2804>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2659–2661; for a review, see:10.1002/(SICI)1521-3773(19981016)37:19<2659::AID-ANIE2659>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 15bE. Guitiàn, D. Pérez, D. Peña in Topics in Organometallic Chemistry, Vol. 14 (Ed.: ), Springer, Weinheim, 2005, pp. 109–146.
- 16
- 16aK. V. Radhakrishnan, E. Yoshikawa, Y. Yamamoto, Tetrahedron Lett. 1999, 40, 7533–7535;
- 16bD. Peña, S. Escudero, D. Pérez, E. Guitián, L. Castedo, J. Am. Chem. Soc. 1999, 121, 5827–5828;
- 16cE. Yoshikawa, Y. Yamamoto, Angew. Chem. 2000, 112, 185–187;
10.1002/(SICI)1521-3757(20000103)112:1<185::AID-ANGE185>3.0.CO;2-N Google ScholarAngew. Chem. Int. Ed. 2000, 39, 173–175;10.1002/(SICI)1521-3773(20000103)39:1<173::AID-ANIE173>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 16dD. Peña, D. Pérez, D. E. Guitiàn, L. Castedo, J. Org. Chem. 2000, 65, 6944–6950;
- 16eN. Chatani, A. Kamitani, M. Oshita, Y. Fukumoto, S. Murai, J. Am. Chem. Soc. 2001, 123, 12686–12687;
- 16fY. Sato, T. Tamura, M. Mori, Angew. Chem. 2004, 116, 2490–2494; Angew. Chem. Int. Ed. 2004, 43, 2436–2440;
- 16gM. Jeganmohan, C. H. Cheng, Org. Lett. 2004, 6, 2821–2824;
- 16hC. H. Cheng, M. Jeganmohan, Synthesis 2005, 1693–1697;
- 16iT. T. Jayanth, M. Jeganmohan, C. H. Cheng, Org. Lett. 2005, 7, 2921–2924;
- 16jJ. L. Henderson, A. S. Edwards, M. F. Greaney, J. Am. Chem. Soc. 2006, 128, 7426–7427;
- 16kZ. J. Liu, R. C. Larock, Angew. Chem. 2007, 119, 2587–2590; Angew. Chem. Int. Ed. 2007, 46, 2535–2538;
- 16lZ. J. Liu, R. C. Larock, Tetrahedron 2007, 63, 347–355;
- 16mY. Sato, T. Tamura, A. Kinbara, M. Mori, Adv. Synth. Catal. 2007, 349, 647–661;
- 16nC. S. Xie, Y. H. Zhang, Z. D. Huang, P. X. Xu, J. Org. Chem. 2007, 72, 5431–5434.
- 17Y. Himeshima, T. Sonoda, H. Kobayashi, Chem. Lett. 1983, 1211–1214.
- 18Z. Liu, X. Zhang, R. C. Larock, J. Am. Chem. Soc. 2005, 127, 15716–15717.
- 19CsF is able to reduce PdII to Pd0, see: M. R. Mason, J. G. Verkade, Organometallics 1992, 11, 2212–2220.
- 20For reduction of the [{(allyl)PdCl}2], see: S. E. Denmark, R. C. Smith, Synlett 2006, 2921–2928; and references therein.
- 21For an exemple of solvent induced divergent behavior, see: E. Yoshikawa, K. V. Radhakrishnan, Y. Yamamoto, J. Am. Chem. Soc. 2000, 122, 7280–7286.
- 22For a review, see: R. E. Gawly, Org. React. 1988, 35, 1–420.
- 23
- 23aE. K. Fields, S. Meyerson, J. Org. Chem. 1966, 31, 3307–3309;
- 23bN. Dennis, A. R. Katritzky, S. K. Parton, J. Chem. Soc. Perkin Trans. 1 1976, 2285–2288;
- 23cD. K. Rayabarapu, K. K. Majumdar, T. Sambaiah, C.-H Cheng, J. Org. Chem. 2001, 66, 3646–3649.
- 24M. Jeganmohan, C.-H. Cheng, Chem. Commun. 2006, 2454–2456.
- 25Microwave- or light-induced transformation of oximes derivatives to phenanthridines or isoquinolines have been reported:
- 25aR. Alonso, P. J. Campos, B. Garcia, M. A. Rodriguez, Org. Lett. 2006, 8, 3521–3523;
- 25bF. Portela-Cubillo, J. S. Scott, J. C. Walton, J. Org. Chem. 2008, 73, 5558–5565.
- 26Very recently, cyclocondensation between arynes and nitriles have been reported under nickel catalysis conditions: J.-C. Hsieh, C.-H. Cheng, Chem. Commun. 2008, 2992–2994.
- 27M. Retboll, A. J. Edwards, A. D. Rae, A. C. Willis, M. A. Bennett, E. Wenger, J. Am. Chem. Soc. 2002, 124, 8348–8360.
- 28Some ketones were prepared according to: P.-A. Enquist, P. Nilsson, M. Larhed, Org. Lett. 2003, 5, 4875–4878.
- 29
- 29aD. Garcia-Cuadrado, A. A. C. Braga, F. Maseras, A. M. Echavarren, J. Am. Chem. Soc. 2006, 128, 1066–1067;
- 29bF. Bonnaterre, M. Bois-Choussy, J. Zhu, Beilstein J. Org. Chem. 2008, 4, 1–6. Note that no annulation product was formed in the case of bis(4-methoxyphenyl)methanone acyloxime.
- 30We thank the referee for suggesting this control experiment; see also:
- 30aT. T. Jayanth, M. Jeganmohan, M.-J. Cheng, S.-Y. Chu, C.-H. Cheng, J. Am. Chem. Soc. 2006, 128, 2232–2233;
- 30bI. Quintana, A. J. Boersma, D. Peña, D. Pérez, E. Guitián, Org. Lett. 2006, 8, 3347–3349.
- 31
- 31aC. M. P. Ferreira, M. Fátina, C. Guesda de Silva, V. Y. Kukushkin, J. J. R. Fraústo da Silva, A. J. L. Pombeiro, J. Chem. Soc. Dalton Trans. 1998, 325–326;
- 31bV. Y. Kukushkin, A. J. L. Pombeiro, Coord. Chem. Rev. 1999, 181, 147–175;
- 31cA. Tillack, P. Arndt, A. Spannenberg, R. Kempe, U. Rosenthal, Z. Anorg. Allg. Chem. 1998, 624, 737–740.
- 32Microwave experiments were conducted by using a Discover microwave reactor from CEM. All experiments were performed in sealed tubes under an argon atmosphere. Microwave irradiation of 300 W was used, the temperature was ramped from room temperature to 150 °C in 1 minute. Once this temperature was reached, the reaction mixture was held at 150 °C for the indicated time. For recent reviews, see:
- 32aC. O. Kappe, Angew. Chem. 2004, 116, 6408–6443;
10.1002/ange.200400655 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 6250–6284;
- 32bA. de La Hoz, A. Diaz-Ortiz, A. Moreno, Chem. Soc. Rev. 2005, 34, 164–178.
- 33For a recent review, see: J. Dupont, C. S. Consorti, J. Spencer, Chem. Rev. 2005, 105, 2527–2572.
- 34
- 34aRef: [18] and
- 34bJ. L. Henderson, A. S. Edwards, M. F. Greaney, Org. Lett. 2007, 9, 5589–5592.
- 35H. Yoshida, K. Tanino, J. Ohshita, A. Kunai, Angew. Chem. 2004, 116, 5162–5165; Angew. Chem. Int. Ed. 2004, 43, 5052–5055.