Isolation of Bicyclopropenylidenes: Derivatives of the Smallest Member of the Fulvalene Family†
Rei Kinjo Dr.
UCR–CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorYutaka Ishida Dr.
UCR–CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorBruno Donnadieu
UCR–CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorGuy Bertrand Prof.
UCR–CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorRei Kinjo Dr.
UCR–CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorYutaka Ishida Dr.
UCR–CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorBruno Donnadieu
UCR–CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorGuy Bertrand Prof.
UCR–CNRS Joint Research Chemistry Laboratory (UMI 2957), Department of Chemistry, University of California, Riverside, CA 92521-0403 (USA), Fax: (+1) 951-827-2725 http://research.chem.ucr.edu/groups/bertrand/guybertrandwebpage/
Search for more papers by this authorFinancial support from the NSF (CHE 0808825) is gratefully acknowledged. We also thank the JSPS for Fellowships (R.K. and Y.I.) and Dr. J. C. Daran for his help with the crystallographic part of this manuscript.
Graphical Abstract
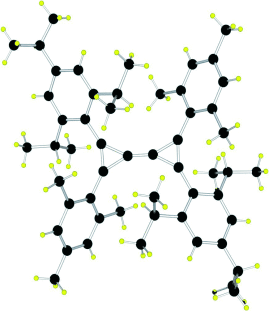
Small and stable: A triafulvalene derivative is indefinitely stable at room temperature both in solution and in the solid state under inert atmosphere. The triafulvalene is synthesized by the magnesium-catalyzed coupling of two bis(chlorocyclopropenyl) derivatives. The two unsaturated three-membered rings are linked together by a carbon–carbon double bond (1.303 Å, see structure), which is so reactive that spontaneous addition of water occurs at room temperature.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200804372_sm_miscellaneous_information.pdf632.1 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. D. Brown, Trans. Faraday Soc. 1949, 45, 296–300.
- 2
- 2aC. Courtot, Ann. Chim. 1915, 4, 157–224;
- 2bC. Courtot, Ann. Chim. 1916, 5, 52–108.
- 3Although the compound was prepared by Courtot, its structure was ascertained in 1971: H. Prinzbach, Pure Appl. Chem. 1971, 28, 281–329.
- 4E. C. Schreiber, E. I. Becker, J. Am. Chem. Soc. 1954, 76, 6125–6127.
- 5N. Ueno, I. Murata, Y. Kitahara, Tetrahedron Lett. 1965, 6, 2967–2970.
10.1016/S0040-4039(01)89242-X Google Scholar
- 6For recent reviews on fulvalenes, see: B. Halton, Eur. J. Org. Chem. 2005, 3391–3414; H. Hopf, Classics in Hydrocarbon Chemistry, Wiley-VCH, Weinheim, 2000.
- 7For recent reviews on tetrathiafulvalenes, see:
- 7aM. Bendikov, F. Wudl, D. F. Perepichka, Chem. Rev. 2004, 104, 4891–4945;
- 7bA. Gorgues, P. Hudhomme, M. Sallé, Chem. Rev. 2004, 104, 5151–5184; See also: P. Batail, Chem. Rev. 2004, 104, 4887–4890.
- 8
- 8aA. P. Scott, I. Agranat, P. U. Biedermann, N. V. Riggs, L. Radom, J. Org. Chem. 1997, 62, 2026–2038;
- 8bC. Läng, M. Mühlebach, M. Neuenschwander, Helv. Chim. Acta 1997, 80, 2124–2136;
- 8cY. Apeloig, R. Boese, B. Halton, A. H. Maulitz, J. Am. Chem. Soc. 1998, 120, 10147–10153;
- 8dR. F. Langler, Aust. J. Chem. 2001, 54, 51–57;
- 8eH. Möllerstedt, M. C. Piqueras, R. Crespo, H. Ottosson, J. Am. Chem. Soc. 2004, 126, 13938–13939;
- 8fG. Ghigo, A. R. M. Shahi, L. Gagliardi, L. M. Solstad, C. J. Cramer, J. Org. Chem. 2007, 72, 2823–2831;
- 8gM. M. Montero-Campillo, J. Rodriguez-Otero, E. M. Cabaleiro-Lago, J. Mol. Model. 2007, 13, 919–926;
- 8hE. Kleinpeter, A. Holzberger, P. Wacker, J. Org. Chem. 2008, 73, 56–65.
- 9R. Neidlein, V. Poignée, W. Kramer, C. Glück, Angew. Chem. 1986, 98, 735–736; Angew. Chem. Int. Ed. Engl. 1986, 25, 731–732.
- 10B. Halton, M. J. Cooney, C. S. Jones, R. Boese, D. Bläser, Org. Lett. 2004, 6, 4017–4020.
- 11It is well recognized that annelation of the cyclopropene ring into an aromatic framework significantly enhances its thermodynamic stability. See for examples:
- 11aB. Halton, P. J. Stang, Synlett 1997, 145–158;
- 11bB. Halton, Chem. Rev. 2003, 103, 1327–1370;
- 11cG. M. Dixon, B. Halton, Eur. J. Org. Chem. 2004, 3707–3713.
- 12E. A. Carter, W. A. Goddard III, J. Phys. Chem. 1986, 90, 998–1001.
- 13
- 13aV. Lavallo, Y. Canac, B. Donnadieu, W. W. Schoeller, G. Bertrand, Science 2006, 312, 722–724;
- 13bV. Lavallo, Y. Ishida, B. Donnadieu, G. Bertrand, Angew. Chem. 2006, 118, 6804–6807;
10.1002/ange.200602701 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6652–6655;
- 13cD. Holschumacher, C. G. Hrib, P. G. Jones, M. Tamm, Chem. Commun. 2007, 3661–3663;
- 13dW. W. Schoeller, G. D. Frey, G. Bertrand, Chem. Eur. J. 2008, 14, 4711–4718.
- 14J. T. DePinto, W. A. deProphetis, J. L. Menke, R. J. McMahon, J. Am. Chem. Soc. 2007, 129, 2308–2315.
- 15
- 15aG. Trinquier, J. P. Malrieu, J. Am. Chem. Soc. 1987, 109, 5303–5315;
- 15bJ. P. Malrieu, G. Trinquier, J. Am. Chem. Soc. 1989, 111, 5916–5921;
- 15cH. Jacobsen, T. Ziegler, J. Am. Chem. Soc. 1994, 116, 3667–3679.
- 16
- 16aR. West, D. C. Zecher, S. W. Tobey, J. Am. Chem. Soc. 1970, 92, 168–172;
- 16bR. West, D. C. Zecher, W. Goyert, J. Am. Chem. Soc. 1970, 92, 149–154.
- 17
- 17aR. Breslow, P. Gal, J. Am. Chem. Soc. 1959, 81, 4747–4748;
- 17bR. Breslow, P. Gal, H. W. Chang, L. J. Altman, J. Am. Chem. Soc. 1965, 87, 5139–5144.
- 18
- 18aA. de Meijere, S. I. Kozhushkov, A. F. Khlebnikov, H. Schill, Top. Curr. Chem. 2000, 207, 89–147;
- 18bA. de Meijere, S. I. Kozhushkov, Chem. Rev. 2006, 106, 4926–4996.
- 19CCDC 693680 (6 a) and 698793 (7 b) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 20Z. Yoshida, Y. Tawara, Tetrahedron Lett. 1971, 12, 3603–3606.
10.1016/S0040-4039(01)97241-7 Google Scholar
- 21Cyclopropenones are notorious for inducing skin sensitivity and dermatitis.