“Blackening” Porphyrins by Conjugation with Quinones†
Correction(s) for this article
-
“Blackening” Porphyrins by Conjugation with Quinones
- Volume 48Issue 14Angewandte Chemie International Edition
- pages: 2442-2442
- First Published online: March 17, 2009
Srinivas Banala Dr.
Institute of Organic Chemistry, University of Innsbruck, 6020 Innsbruck (Austria), Fax: (+43) 512-507-2892
Search for more papers by this authorThomas Rühl Dr.
Institute of Organic Chemistry, University of Innsbruck, 6020 Innsbruck (Austria), Fax: (+43) 512-507-2892
Search for more papers by this authorKlaus Wurst Dr.
Institute of General, Inorganic and Theoretical Chemistry, University of Innsbruck (Austria)
Search for more papers by this authorBernhard Kräutler Prof. Dr.
Institute of Organic Chemistry, University of Innsbruck, 6020 Innsbruck (Austria), Fax: (+43) 512-507-2892
Search for more papers by this authorSrinivas Banala Dr.
Institute of Organic Chemistry, University of Innsbruck, 6020 Innsbruck (Austria), Fax: (+43) 512-507-2892
Search for more papers by this authorThomas Rühl Dr.
Institute of Organic Chemistry, University of Innsbruck, 6020 Innsbruck (Austria), Fax: (+43) 512-507-2892
Search for more papers by this authorKlaus Wurst Dr.
Institute of General, Inorganic and Theoretical Chemistry, University of Innsbruck (Austria)
Search for more papers by this authorBernhard Kräutler Prof. Dr.
Institute of Organic Chemistry, University of Innsbruck, 6020 Innsbruck (Austria), Fax: (+43) 512-507-2892
Search for more papers by this authorThe authors thank Thomas Müller, Simone Moser, and Prof. Ernst Ellmerer for mass and NMR spectra. The work in Innsbruck was supported by the Austrian National Science Foundation (FWF project no. P-17437) and the Tyrolean Science Foundation (project no. UNI 404/22).
Graphical Abstract
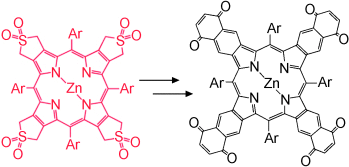
From red to black porphyrins: “Black” porphyrins absorb visible light effectively at all wavelengths and are available by conjugation of four benzoquinone units to a porphyrin core. These robust molecular components are likely to be useful in optoelectronic devices as they absorb a large fraction of the visible light.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200804143_sm_miscellaneous_information.pdf256.7 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. R. Battersby, Science 1994, 264, 1551–1557.
- 2B. Kräutler, Chimia 1987, 41, 277–292.
- 3M. Gouterman in The Porphyrins, Vol. III (Ed.: ), Academic Press, New York, 1978, pp. 1–165.
- 4F. R. Hopf, D. G. Whitten in The Porphyrins, Vol. II (Ed.: ), Academic Press, New York, 1978, pp. 161–195.
10.1016/B978-0-12-220102-8.50013-0 Google Scholar
- 5K. M. Smith in The Porphyrin Handbook, Vol. 1 (Eds.: ), Elsevier Science, Oxford, 2000, pp. 1–43.
- 6H. Fischer, K. Zeile, Justus Liebigs Ann. Chem. 1929, 468, 98–116.
- 7R. B. Woodward, Angew. Chem. 1960, 72, 651–662.
- 8H. Zollinger, Farbe. Eine multidisziplinäre Betrachtung, Wiley-VCH, Zürich, 2005.
- 9 The Porphryin Handbook, Vol. 4, (Eds.: ), Academic Press, San Diego, 2000, pp. 1–339.
- 10K. M. Kadish, E. V. Caemelbecke, G. Royal in The Porphyrin Handbook, Vol. 8 (Eds.: ), Academic Press, San Diego, 2000, pp. 1–114.
- 11H. Mustroph, M. Stollenwerk, V. Bressau, Angew. Chem. 2006, 118, 2068–2087;
10.1002/ange.200502820 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 2016–2035.
- 12R. L. Carroll, C. B. Gorman, Angew. Chem. 2002, 114, 4556–4579;
10.1002/1521-3757(20021202)114:23<4556::AID-ANGE4556>3.0.CO;2-W Google ScholarAngew. Chem. Int. Ed. 2002, 41, 4378–4400.10.1002/1521-3773(20021202)41:23<4378::AID-ANIE4378>3.0.CO;2-A CAS PubMed Web of Science® Google Scholar
- 13J. J. Müller, U. H. F. Bunz, Functional Organic Materials, Wiley-VCH, Weinheim, 2007.
10.1002/9783527610266 Google Scholar
- 14M. Hoffmann, C. J. Wilson, B. Odell, H. L. Anderson, Angew. Chem. 2007, 119, 3183–3186;
10.1002/ange.200604601 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3122–3125.
- 15R. Pandey, G. Zheng in The Porphyrin Handbook, Vol. 6 (Eds.: ), Elsevier Science, Oxford, 2000, pp. 158–230.
- 16 The Porphryin Handbook, Vol. 1–10 (Eds.: ), Academic Press, San Diego, 2000.
- 17 The Porphryin Handbook, Vol. 11–20 (Eds.: ), Academic Press, San Diego, 2003.
- 18N. Robertson, Angew. Chem. 2008, 120, 1028–1030; Angew. Chem. Int. Ed. 2008, 47, 1012–1014.
- 19F. J. Dyson, A Many-Colored Glass—Reflections on the Place of Life in the Universe, University of Virginia Press, Charlottesville, 2007.
- 20B. Kräutler, C. S. Sheehan, A. Rieder, Helv. Chim. Acta 2000, 83, 583–591.
10.1002/(SICI)1522-2675(20000315)83:3<583::AID-HLCA583>3.0.CO;2-A CAS Web of Science® Google Scholar
- 21A. Rieder, B. Kräutler, J. Am. Chem. Soc. 2000, 122, 9050–9051.
- 22D. Gust, T. A. Moore in The Porphyrin Handbook, Vol. 8 (Eds.: ), Elsevier Science, Oxford, 2000, pp. 153–190.
- 23A. J. Bard, L. R. Faulkner, Electrochemical Methods. Fundamentals and Applications, Wiley, New York, 1980.
- 24H. Lund, M. M. Baizer, Organic Electrochemistry, 3rd ed., Marcel Dekker, New York, 1991.
- 25Other recently prepared mononuclear porphyrinoids with extended conjugated π systems are all deduced or described as colored and display a characteristic (sometimes split) Soret band and weaker Q bands; see, for example,
- 25aT. Lash in The Porphyrin Handbook, Vol. 2 (Eds.: ), Elsevier Science, Oxford, 2000, pp. 125–199;
- 25bT. Okujima, Y. Hashimoto, G. Jin, H. Yamada, N. Ono, Heterocycles 2009, DOI: .
- 26S. Niyogi, E. Bekyarova, M. E. Itkis, J. L. McWilliams, M. A. Hamon, R. C. Haddon, J. Am. Chem. Soc. 2006, 128, 7720–7721.
- 27C. Dekker, Phys. Today 1999, 52, 22–28.
- 28J.-J. Cid, J.-H. Yum, S.-R. Jang, M. K. Nazeeruddin, E. Martinez-Ferrero, E. Palomares, J. Ko, M. Grätzel, T. Torres, Angew. Chem. 2007, 119, 8510–8514;
10.1002/ange.200703106 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8358–8362.
- 29E. Kaxiras, A. Tsolakidis, G. Zonis, S. Meng, Phys. Rev. Lett. 2006, 97, 218102.