Planar Tetranuclear and Dumbbell-Shaped Octanuclear Palladium Complexes with Bridging Silylene Ligands†
Tetsuyuki Yamada
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 (Japan), Fax: (+81) 45-924-5224
Search for more papers by this authorAkane Mawatari
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 (Japan), Fax: (+81) 45-924-5224
Search for more papers by this authorMakoto Tanabe Dr.
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 (Japan), Fax: (+81) 45-924-5224
Search for more papers by this authorKohtaro Osakada Prof.
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 (Japan), Fax: (+81) 45-924-5224
Search for more papers by this authorTomoaki Tanase Prof.
Department of Chemistry, Faculty of Science, Nara Women's University, Kitauoya-higashi-machi, Nara 630-8506 (Japan)
Search for more papers by this authorTetsuyuki Yamada
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 (Japan), Fax: (+81) 45-924-5224
Search for more papers by this authorAkane Mawatari
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 (Japan), Fax: (+81) 45-924-5224
Search for more papers by this authorMakoto Tanabe Dr.
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 (Japan), Fax: (+81) 45-924-5224
Search for more papers by this authorKohtaro Osakada Prof.
Chemical Resources Laboratory, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8503 (Japan), Fax: (+81) 45-924-5224
Search for more papers by this authorTomoaki Tanase Prof.
Department of Chemistry, Faculty of Science, Nara Women's University, Kitauoya-higashi-machi, Nara 630-8506 (Japan)
Search for more papers by this authorThis work was financially supported by Grants-in-Aid for Scientific Research for Young Chemists (No. 19750043), for Scientific Research (No. 1925008), and for Scientific Research on Priority Areas (No. 19027018), from the Ministry of Education, Culture, Sport, Science, and Technology Japan. We thank Rieko Kobayashi (Tokyo Institute of Technology) for experimental assistance.
Graphical Abstract
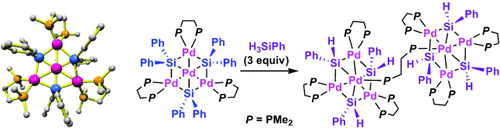
An uncommon structure: The novel title compounds (see scheme) were prepared and fully characterized. The tetranuclear complex contains a hexagonal Pd4Si3 core involving one central Pd atom, three outer Pd atoms, and three bridging Si atoms within the same plane, whereas the dumbbell-shaped octanuclear Pd complex is composed of two Pd4Si3 groups bridged by a diphosphine ligand.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200804728_sm_miscellaneous_information.pdf717 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aK. Yamamoto, H. Okinoshima, M. Kumada, J. Organomet. Chem. 1970, 23, C 7–C8;
- 1bK. Yamamoto, H. Okinoshima, M. Kumada, J. Organomet. Chem. 1971, 27, C 31–C32.
- 2
- 2aS. D. Grumbine, T. D. Tilley, J. Am. Chem. Soc. 1993, 115, 7884–7885;
- 2bG. P. Mitchell, T. D. Tilley, Angew. Chem. 1998, 110, 2602–2605;
10.1002/(SICI)1521-3757(19980918)110:18<2602::AID-ANGE2602>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2524–2526;10.1002/(SICI)1521-3773(19981002)37:18<2524::AID-ANIE2524>3.0.CO;2-H CAS PubMed Web of Science® Google Scholar
- 2cJ. D. Feldman, G. P. Mitchell, J.-O. Nolte, T. D. Tilley, J. Am. Chem. Soc. 1998, 120, 11184–11185;
- 2dJ. D. Feldman, G. P. Mitchell, J.-O. Nolte, T. D. Tilley, Can. J. Chem. 2003, 81, 1127–1136.
- 3
- 3aP. D. Lickiss, Chem. Soc. Rev. 1992, 21, 271–279;
- 3bC. Zybill, H. Handwerker, H. Friedrich, Adv. Organomet. Chem. 1994, 36, 229–281;
- 3cM. Okazaki, H. Tobita, H. Ogino, Dalton Trans. 2003, 493–506;
- 3dR. Waterman, P. G. Hayes, T. D. Tilley, Acc. Chem. Res. 2007, 40, 712–719.
- 4
- 4aH. Ogino, H. Tobita, Adv. Organomet. Chem. 1998, 42, 223–290;
- 4bJ. Y. Corey, J. Braddock-Wilking, Chem. Rev. 1999, 99, 175–292;
- 4cP. Braunstein, N. M. Boag, Angew. Chem. 2001, 113, 2493–2499;
10.1002/1521-3757(20010702)113:13<2493::AID-ANGE2493>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2001, 40, 2427–2433;10.1002/1521-3773(20010702)40:13<2427::AID-ANIE2427>3.0.CO;2-T CAS PubMed Web of Science® Google Scholar
- 4dK. Osakada, M. Tanabe, Bull. Chem. Soc. Jpn. 2005, 78, 1887–1898;
- 4eS. Shimada, M. Tanaka, Coord. Chem. Rev. 2006, 250, 991–1011.
- 5M. Auburn, M. Ciriano, J. A. K. Howard, M. Murray, N. J. Pugh, J. L. Spencer, F. G. A. Stone, P. Woodward, J. Chem. Soc. Dalton Trans. 1980, 659–666.
- 6
- 6aA. Brookes, S. A. R. Knox, F. G. A. Stone, J. Chem. Soc. A 1971, 3469–3471;
- 6bS. G. Anema, S. K. Lee, K. M. Mackay, B. K. Nicholson, J. Organomet. Chem. 1993, 444, 211–218;
- 6cC. Evans, G. J. Harfoot, J. S. McIndoe, C. J. McAdam, K. M. Mackay, B. K. Nicholson, B. H. Robinson, M. L. Van Tiel, J. Chem. Soc. Dalton Trans. 2002, 4678–4683;
- 6dC. Evans, B. K. Nicholson, J. Organomet. Chem. 2003, 665, 95–100.
- 7
- 7a Metal–Metal Bonds and Clusters in Chemistry and Catalysis (Ed.: ), Plenum, New York, 1990;
- 7bD. A. Admas, F. A. Cotton, Catalysis by Di- and Polynuclear Metal Cluster Complexes, Wiley, New York, 1998;
- 7c Clusters and Colloids (Ed.: ), VCH, Weinheim, 1994.
- 8
- 8aJ. Braddock-Wilking, J. Y. Corey, K. Dill, N. P. Rath, Organometallics 2002, 21, 5467–5469;
- 8bJ. Braddock-Wilking, J. Y. Corey, L. M. French, E. Choi, V. J. Speedie, M. F. Rutherford, S. Yao, H. Xu, N. P. Rath, Organometallics 2006, 25, 3974–3988.
- 9K. Osakada, M. Tanabe, T. Tanase, Angew. Chem. 2000, 112, 4219–4221;
10.1002/1521-3757(20001117)112:22<4219::AID-ANGE4219>3.0.CO;2-3 Google ScholarAngew. Chem. Int. Ed. 2000, 39, 4053–4055.10.1002/1521-3773(20001117)39:22<4053::AID-ANIE4053>3.0.CO;2-Z CAS PubMed Web of Science® Google Scholar
- 10We reported the complex as the first example of a Pt0 complex with Si ligands, but, in fact, a mononuclear Pt0 complex with a nonbridging silylene ligand had been found earlier. See ref. [2b].
- 11W. Chen, S. Shimada, M. Tanaka, Science 2002, 295, 308–310.
- 12M. Tanabe, T. Yamada, K. Osakada, Organometallics 2003, 22, 2190–2192.
- 13M. Tanabe, A. Mawatari, K. Osakada, Organometallics 2007, 26, 2937–2940.
- 14Crystallographic data for (2)⋅C6H14: C60H92P6Pd4Si3; Mr=1509.09; crystal size 0.18×0.33×0.65 mm3; monoclinic; P21/c; a=13.614(2), b=27.398(4), c=18.687(3) Å; β=99.218(2)°; V=6880(2) Å3; Z=4; ρcalcd=1.457 g cm−3; μ=1.2552 mm−1; F(000)=3072, T=133 K; Rigaku Saturn CCD area detector equipped with monochromated MoKα radiation (λ=0.71070 Å); variables=750; 50 104 reflections measured (2θmax=55.0°); 15 569 independent reflections (Rint=0.034); R1=0.0295; wR2=0.0730; GOF=1.021. Crystallographic data for (4)⋅2 C6H14: C90H176P14Pd8Si6; Mr=2711.73; crystal size 0.43×0.68×0.75 mm3; monoclinic; P21/c; a=14.231(2), b=18.514(2), c=22.498(3) Å; β=98.047(2)°; V=5869(2) Å3; Z=2; ρcalcd=1.534 g cm−3; μ=1.4874 mm−1; F(000)=2756; T=133 K; Rigaku Saturn CCD area detector equipped with monochromated MoKα radiation (λ=0.71070 Å); variables=629; 39 847 reflections measured (2θmax=55.0°); 13 084 independent reflections(Rint=0.024); R1=0.0413; wR2=0.1177; GOF=0.980. CCDC 702935 (2) and 702937 (4) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 15C. Watanabe, T. Iwamoto, C. Kabuto, M. Kira, Angew. Chem. 2008, 120, 5466–5469; Angew. Chem. Int. Ed. 2008, 47, 5386–5389.
- 16D. Kovala-Demertzi in Metal Clusters in Chemistry, Vol. 1 (Eds.: ), Wiley-VCH, Weinheim, 1999, pp. 399–416.
10.1002/9783527618316.ch1u Google Scholar
- 17Y. Apeloig in The Chemistry of Organic Silicon Compounds (Eds.: ), Wiley, Chichester, 1989.
- 18S. E. Kabir, A. K. Raha, M. R. Hassan, B. K. Nicholson, E. Rosenberg, A. Sharmin, L. Salassa, Dalton Trans. 2008, 4212–4219.
- 19
- 19aR. J. Puddephatt, Polyhedron 1990, 9, 2767–2802;
- 19bP. D. Harvey, J. Cluster Sci. 1993, 4, 377–402;
- 19cD. Imhof, L. M. Venanzi, Chem. Soc. Rev. 1994, 23, 185–193;
- 19dA. D. Burrows, D. M. P. Mingos, Coord. Chem. Rev. 1996, 154, 19–69;
- 19eW. Schuh, P. Braunstein, M. Bénard, M.-M. Rohmer, R. Welter, J. Am. Chem. Soc. 2005, 127, 10250–10258.
- 20S. Shimada, Y.-H. Li, Y.-K. Choe, M. Tanaka, M. Bao, T. Uchimaru, Proc. Natl. Acad. Sci. USA 2007, 104, 7758–7763.
- 21W.-D. Wang, R. Eisenberg, Organometallics 1992, 11, 908–912.
- 22M. Ochiai, H. Hashimoto, H. Tobita, Angew. Chem. 2007, 119, 8340–8342;
10.1002/ange.200703154 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8192–8194.
- 23P. G. Hayes, C. Beddie, M. B. Hall, R. Waterman, T. D. Tilley, J. Am. Chem. Soc. 2006, 128, 428–429.
- 24
- 24aR. S. Simons, J. C. Gallucci, C. A. Tessier, W. J. Youngs, J. Organomet. Chem. 2002, 654, 224–228;
- 24bJ. D. Feldman, J. C. Peters, T. D. Tilley, Organometallics 2002, 21, 4065–4075.
- 25S. Shimada, M. L. N. Rao, M. Tanaka, Organometallics 1999, 18, 291–293.