Self-Assembled Bidentate Ligands for the Nickel-Catalyzed Hydrocyanation of Alkenes†
Michiel de Greef Dr.
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg i. Brsg. (Germany), Fax: (+49) 761-203-8715
Search for more papers by this authorBernhard Breit Prof. Dr.
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg i. Brsg. (Germany), Fax: (+49) 761-203-8715
Search for more papers by this authorMichiel de Greef Dr.
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg i. Brsg. (Germany), Fax: (+49) 761-203-8715
Search for more papers by this authorBernhard Breit Prof. Dr.
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg i. Brsg. (Germany), Fax: (+49) 761-203-8715
Search for more papers by this authorThis work was supported by the Alexander von Humboldt-Foundation (fellowship to M.d.G.), the DFG (IRTG 1038) and the Krupp Foundation.
Graphical Abstract
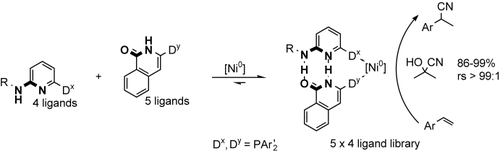
Well-stocked library: A catalyst with interesting activity, regioselectivity (rs), and functional-group tolerance could be identified from a 5×4 library of isoquinolone- or aminopyridine-derived self-assembled bidentate ligands. These ligands, which self-assemble through hydrogen bonding, form complexes with nickel(0) that appeared to be promising catalysts for the hydrocyanation of styrene (see scheme).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200805092_sm_miscellaneous_information.pdf155.1 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1P. Pollak, G. Romeder, F. Hagedorn, H. Gelbke in Ullman's Encyclopedia of Industrial Chemistry, Vol. A17, Wiley-VCH, Weinheim, 1985, pp. 363–376.
- 2K. Huthmacher, S. Krill in Applied Homogeneous Catalysis with Organometallic Compounds, Vol. 1 (Eds: ), Wiley-VCH, Weinheim, 2002, pp. 465–486.
- 3M. Kranenburg, P. C. J. Kamer, P. W. N. M. van Leeuwen, D. Vogt, W. Keim, J. Chem. Soc. Chem. Commun. 1995, 2177–2178.
- 4W. Goertz, P. C. J. Kamer, P. W. N. M. van Leeuwen, D. Vogt, Chem. Commun. 1997, 1521–1522.
- 5W. Goertz, W. Keim, D. Vogt, U. Englert, M. D. K. Boele, L. A. van der Veen, P. C. J. Kamer, P. W. N. M. van Leeuwen, J. Chem. Soc. Dalton Trans. 1998, 2981–2988.
- 6E. Burello, P. Marion, J.-C. Galland, A. Chamard, G. Rothenberg, Adv. Synth. Catal. 2005, 347, 803–810.
- 7L. Bini, C. Müller, J. Wilting, L. von Chrzanowski, A. L. Spek, D. Vogt, J. Am. Chem. Soc. 2007, 129, 12622–12623.
- 8A. Acosta-Ramírez, A. Flores-Gaspar, M. Muñoz-Hernández, A. Arévalo, W. D. Jones, J. J. García, Organometallics 2007, 26, 1712–1720.
- 9Z. Freixa, P. W. N. M. van Leeuwen, Dalton Trans. 2003, 1890–1901.
- 10C. A. Tolman, R. J. McKinney, W. C. Seidel, J. D. Druliner, W. R. Stevens, Adv. Catal. 1985, 33, 1–46.
- 11B. Breit, Angew. Chem. 2005, 117, 6976–6986;
10.1002/ange.200501798 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 6816–6825.
- 12J. D. Watson, F. H. C. Crick, Nature 1953, 171, 964–967.
- 13For alternative approaches to bidentate ligands through self-assembly, see Ref. [11] and:
- 13aJ. M. Takacs, D. S. Reddy, S. A. Moteki, D. Wu, H. Palencia, J. Am. Chem. Soc. 2004, 126, 4494–4495;
- 13bM. J. Wilkinson, P. W. N. M. van Leeuwen, J. N. H. Reek, Org. Biomol. Chem. 2005, 3, 2371–2383;
- 13cV. F. Slagt, M. Röder, P. C. J. Kamer, P. W. N. M. van Leeuwen, J. N. H. Reek, J. Am. Chem. Soc. 2004, 126, 4056–4057;
- 13dV. F. Slagt, P. W. N. M. van Leeuwen, J. N. H. Reek, Angew. Chem. 2003, 115, 5777–5781;
10.1002/ange.200352525 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 5619–5623;
- 13eV. F. Slagt, P. W. N. M. van Leeuwen, J. N. H. Reek, Chem. Commun. 2003, 2474–2475.
- 14
- 14aB. Breit, W. Seiche, J. Am. Chem. Soc. 2003, 125, 6608–6609;
- 14bB. Breit, W. Seiche, Angew. Chem. 2005, 117, 1666–1669;
10.1002/ange.200462499 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 1640–1643;
- 14cC. Waloch, J. Wieland, M. Keller, B. Breit, Angew. Chem. 2007, 119, 3097–3099;
10.1002/ange.200605202 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3037–3039.
- 15F. Chevallier, B. Breit, Angew. Chem. 2006, 118, 1629–1632;
10.1002/ange.200503826 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 1599–1602.
- 16M. Weis, C. Waloch, W. Seiche, B. Breit, J. Am. Chem. Soc. 2006, 128, 4188–4189.
- 17T. Šmejkal, B. Breit, Organometallics 2007, 26, 2461–2464.
- 18A. L. Casalnuovo, T. V. RajanBabu, T. A. Ayers, T. H. Warren, J. Am. Chem. Soc. 1994, 116, 9869–9882.
- 19W. Goertz, P. C. J. Kamer, P. W. N. M. van Leeuwen, D. Vogt, Chem. Eur. J. 2001, 7, 1614.
10.1002/1521-3765(20010417)7:8<1614::AID-CHEM16140>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 20J. Wilting, M. Janssen, C. Müller, D. Vogt, J. Am. Chem. Soc. 2006, 128, 11374–11375.
- 21B. Saha, T. V. RajanBabu, Org. Lett. 2006, 8, 4657–4659.
- 22J. Wilting, M. Janssen, C. Müller, M. Lutz, A. L. Spek, D. Vogt, Adv. Synth. Catal. 2007, 349, 350–356.