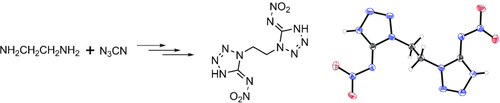
Energetic Mono-, Di-, and Trisubstituted Nitroiminotetrazoles†
Young-Hyuk Joo Dr.
Department of Chemistry, University of Idaho, Moscow, ID 83844-2343 (USA), Fax: (+1) 208-885-9146
Search for more papers by this authorJean'ne M. Shreeve Prof. Dr.
Department of Chemistry, University of Idaho, Moscow, ID 83844-2343 (USA), Fax: (+1) 208-885-9146
Search for more papers by this authorYoung-Hyuk Joo Dr.
Department of Chemistry, University of Idaho, Moscow, ID 83844-2343 (USA), Fax: (+1) 208-885-9146
Search for more papers by this authorJean'ne M. Shreeve Prof. Dr.
Department of Chemistry, University of Idaho, Moscow, ID 83844-2343 (USA), Fax: (+1) 208-885-9146
Search for more papers by this authorWe gratefully acknowledge the support of the DTRA (HDTRA1-07-1-0024), the NSF (CHE-0315275), and the ONR (N00014-06-1-1032). We are grateful to Dr. D. A. Parrish, Naval Research Laboratory (NRL), for determining the single-crystal X-ray structures.
Graphical Abstract
A bundle of energy: The title compounds were synthesized in good yield from aminotetrazoles (obtained from the reaction of cyanogen azide with primary amines) by treatment with 100 % nitric acid and were fully characterized by spectroscopic methods, elemental analysis, and in some cases X-ray diffraction (see example; N blue, O red). The heats of formation of these energetic materials were calculated, as well as their detonation pressures and velocities.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200804755_sm_miscellaneous_information.pdf628.6 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews, see:
- 1aR. P. Singh, R. D. Verma, D. T. Meshri, J. M. Shreeve, Angew. Chem. 2006, 118, 3664–3682; Angew. Chem. Int. Ed. 2006, 45, 3584–3601;
- 1bG. Steinhauser, T. M. Klapötke, Angew. Chem. 2008, 120, 3376–3394;
10.1002/ange.200704510 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3330–3347;
- 1cR. P. Singh, H. Gao, D. T. Meshri, J. M. Shreeve in High Energy Density Materials (Ed.: ), Springer, Berlin, 2007, pp. 35–83;
- 1dT. M. Klapötke in High Energy Density Materials (Ed.: ), Springer, Berlin, 2007, pp. 85–122.
- 2H. H. Klause in Energetic Materials (Ed.: ), VCH, Weinheim, 2005, pp. 1–25.
10.1002/3527603921.ch1 Google Scholar
- 3N. Kubota, Propellants and Explosives, VCH, Weinheim, 2007.
10.1002/9783527610105 Google Scholar
- 4
- 4aT. M. Klapötke, J. Stierstorfer, Helv. Chim. Acta 2007, 90, 2132–2150;
- 4bT. M. Klapötke, H. Radies, J. Stierstorfer, Z. Naturforsch. B. 2007, 62, 1343–1352;
- 4cG. Geisberger, T. M. Klapötke, J. Stierstorfer, Eur. J. Inorg. Chem. 2007, 4743–4750;
- 4dT. M. Klapötke, J. Stierstorfer, A. U. Wallek, Chem. Mater. 2008, 20, 4519–4530;
- 4eH. Gao, Y. Huang, C. Ye, B. Twamley, J. M. Shreeve, Chem. Eur. J. 2008, 14, 5596–5603;
- 4fH. Xue, H. Gao, B. Twamley, J. M. Shreeve, Chem. Mater. 2007, 19, 1731–1739.
- 5
- 5aS. V. Levchik, A. I. Balabanovich, O. A. Ivashkevich, A. I. Lesnikovich, P. N. Gaponik, L. Costa, Thermochim. Acta 1993, 225, 53–65;
- 5bA. I. Lesnikovich, O. A. Ivashkevich, S. V. Levchik, A. I. Balabanovich, P. N. Gaponik, A. A. Kulak, Thermochim. Acta 2002, 388, 233–251.
- 6aT. E. O'Connor, G. Fleming, J. Reilly, J. Soc. Chem. Ind. London Trans. Commun. 1949, 68, 309–310;
- 6bE. Lieber, E. Sherman, R. A. Henry, J. Cohen, J. Am. Chem. Soc. 1951, 73, 2327–2329;
- 6cJ. A. Garrison, R. M. Herbst, J. Org. Chem. 1957, 22, 278–283.
- 7
- 7aF. D. Marsh, M. E. Hermes, J. Am. Chem. Soc. 1964, 86, 4506–4507;
- 7bF. D. Marsh, J. Org. Chem. 1972, 37, 2966–2969;
- 7cJ. E. McMurry, A. P. Coppolino, J. Org. Chem. 1973, 38, 2821–2827;
- 7dK. Banert, Y.-H. Joo, T. Rüffer, B. Walfort, H. Lang, Angew. Chem. 2007, 119, 1187–1190;
10.1002/ange.200603960 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1168–1171.
- 8aY.-H. Joo, J. M. Shreeve, Org. Lett. 2008, 10, 4665–4667;
- 8bY.-H. Joo, B. Twamley, S. Garg, J. M. Shreeve, Angew. Chem. 2008, 120, 6332–6335; Angew. Chem. Int. Ed. 2008, 47, 6236–6239.
- 9An alternate method with 70 % HNO3 was also used for the synthesis of nitroiminotetrazoles 4 and 5. The reaction mixture was stirred for 3 days and then dried in air, and the white solid product was recrystallized from water. However, the products were obtained by this method in just 30–34 % yield.
- 10The 1-substituted aminotetrazole (2 mmol) was added in small portions to 100 % HNO3 (10 mL) at 0 °C. The reaction mixture was stirred at ambient temperature for 18 h and then poured into ice water (20 g) and stirred for a further 3 h. The product was precipitated, filtered, washed with water, and dried in air at room temperature.
- 11Crystallographic data for 1: C3H6N6O3: Mr=174.14; crystal size: 0.88×0.48×0.34 mm3; triclinic, P
 , a=7.1095(13), b=7.1116(13), c=7.8928(15) Å, α=89.235(2), β=66.303(2), γ=66.804(2)°, V=330.86(11) Å3, Z=2, 2θmax=56.6°, ρcalcd=1.748 mg m−3, μ=0.153 mm−1, F(000)=180, R1=0.0393 for 1475 observed (I>2σI) reflections and 0.0419 for all 1616 reflections, goodness-of-fit=1.065, 110 parameters. Crystallographic data for 4: C4H6N12O4: Mr=286.21; crystal size: 0.25×0.12×0.11 mm3; monoclinic, P21/n, a=8.182(3), b=6.614(2), c=10.463(3) Å, α=90, β=112.380(4), γ=90°, V=523.6(3) Å3, Z=2, 2θmax=56.6°, ρcalcd=1.815 mg m−3, μ=0.159 mm−1, F(000)=292, R1=0.0808 for 1124 observed (I>2σI) reflections and 0.0868 for all 1265 reflections, goodness-of-fit=1.228, 91 parameters. CCDC 703780 (1) and CCDC 703781 (4) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif..
, a=7.1095(13), b=7.1116(13), c=7.8928(15) Å, α=89.235(2), β=66.303(2), γ=66.804(2)°, V=330.86(11) Å3, Z=2, 2θmax=56.6°, ρcalcd=1.748 mg m−3, μ=0.153 mm−1, F(000)=180, R1=0.0393 for 1475 observed (I>2σI) reflections and 0.0419 for all 1616 reflections, goodness-of-fit=1.065, 110 parameters. Crystallographic data for 4: C4H6N12O4: Mr=286.21; crystal size: 0.25×0.12×0.11 mm3; monoclinic, P21/n, a=8.182(3), b=6.614(2), c=10.463(3) Å, α=90, β=112.380(4), γ=90°, V=523.6(3) Å3, Z=2, 2θmax=56.6°, ρcalcd=1.815 mg m−3, μ=0.159 mm−1, F(000)=292, R1=0.0808 for 1124 observed (I>2σI) reflections and 0.0868 for all 1265 reflections, goodness-of-fit=1.228, 91 parameters. CCDC 703780 (1) and CCDC 703781 (4) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif..
- 12Gaussian 03 (Revision D.01), M. J. Frisch et al.; see the Supporting Information.
- 13
- 13aL. E. Fried, K. R. Glaesemann, W. M. Howard, P. C. Souers, CHEETAH 4.0 User's Manual, Lawrence Livermore National Laboratory, 2004;
- 13bS. Bastea, L. E. Fried, K. R. Glaesemann, W. M. Howard, P. C. Souers, P. A. Vitello, CHEETAH 5.0 User's Manual, Lawrence Livermore National Laboratory, 2007.
- 14
- 14awww.bam.de.
- 14bA portion of nitroiminotetrazoles 1–9 (20 mg) was subjected to a drop-hammer test, in which a 5 or 10 kg weight was dropped. For the categorization of impact sensitivities (insensitive: >40 J; less sensitive: ≥35 J; sensitive: ≥4 J; very sensitive: ≤3 J), see: J. C. Galvez-Ruiz, G. Holl, K. Karaghiosoff, T. M. Klapötke, K. Loehnwitz, P. Mayer, H. Noeth, K. Polborn, C. J. Rohbogner, M. Suter, J. J. Weigand, Inorg. Chem. 2005, 44, 4237–4253.