A Highly Strained Planar-Chiral Platinacycle for Catalytic Activation of Internal Olefins in the Friedel–Crafts Alkylation of Indoles†
Haoxi Huang Dr.
Laboratorium für Organische Chemie, ETH Zürich, Wolfgang-Pauli-Str. 10, 8093 Zürich (Switzerland)
Search for more papers by this authorRené Peters Prof. Dr.
Institut für Organische Chemie, Universität Stuttgart, Pfaffenwaldring 55, 70569 Stuttgart (Germany), Fax: (+49) 711-685-64321
Laboratorium für Organische Chemie, ETH Zürich, Wolfgang-Pauli-Str. 10, 8093 Zürich (Switzerland)
Search for more papers by this authorHaoxi Huang Dr.
Laboratorium für Organische Chemie, ETH Zürich, Wolfgang-Pauli-Str. 10, 8093 Zürich (Switzerland)
Search for more papers by this authorRené Peters Prof. Dr.
Institut für Organische Chemie, Universität Stuttgart, Pfaffenwaldring 55, 70569 Stuttgart (Germany), Fax: (+49) 711-685-64321
Laboratorium für Organische Chemie, ETH Zürich, Wolfgang-Pauli-Str. 10, 8093 Zürich (Switzerland)
Search for more papers by this authorThis work was financially supported by the ETH research grant TH-01/07-1 and F. Hoffmann-La Roche. We thank Priv.-Doz. Dr. Martin Karpf and Dr. Paul Spurr (both from F. Hoffmann-La Roche, Synthesis and Process Research, Basel) for carefully reading this manuscript and Paul Seiler and Dr. W. Bernd Schweizer (both from ETHZ) for X-ray crystal structure analysis.
Graphical Abstract
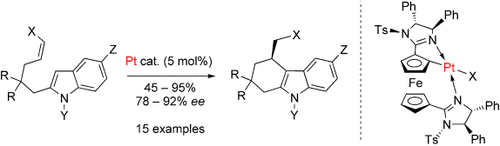
Activation by deformation: A planar-chiral platinacycle readily prepared by diastereoselective cycloplatination enables the enantioselective intramolecular hydroarylation of indoles having disubstituted Z olefins (see scheme; Ts=p-tolylsulfonyl). Sufficient activity is achieved by a combination of highly strained catalyst geometry and accelerated olefin coordination. This application represents the first highly enantioselective reaction catalyzed by a platinacycle.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_200804944_sm_miscellaneous_information.pdf2.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. Fürstner, P. W. Davies, Angew. Chem. 2007, 119, 3478;
10.1002/ange.200604335 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3410;
- 1bA. R. Chianese, S. J. Lee, M. R. Gagné, Angew. Chem. 2007, 119, 4118;
10.1002/ange.200603954 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4042;
- 1cselected example of recent asymmetric applications: C. A. Mullen, A. N. Campbell, M. R. Gagné, Angew. Chem. 2008, 120, 6100; Angew. Chem. Int. Ed. 2008, 47, 6011.
- 2A. S. K. Hashmi, Chem. Rev. 2007, 107, 3180.
- 3F. P. Fanizzi, F. P. Intini, L. Maresca, G. Natile, J. Chem. Soc. Dalton Trans. 1992, 309.
- 4
- 4aM. E. Weiss, D. F. Fischer, Z.-q. Xin, S. Jautze, W. B. Schweizer, R. Peters, Angew. Chem. 2006, 118, 5823;
10.1002/ange.200601731 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5694;
- 4bD. F. Fischer, Z.-q. Xin, R. Peters, Angew. Chem. 2007, 119, 7848; Angew. Chem. Int. Ed. 2007, 46, 7704;
- 4cZ.-q. Xin, D. F. Fischer, R. Peters, Synlett 2008, 1495;
- 4dR. Peters, Z.-q. Xin, D. F. Fischer, W. B. Schweizer, Organometallics 2006, 25, 2917; for the first preparation of chiral ferrocenyl imidazolines, see:
- 4eR. Peters, D. F. Fischer, Org. Lett. 2005, 7, 4137; for selected recent applications of imidazolines in asymmetric catalysis, see for example:
- 4fS. Bhor, G. Anilkumar, M. K. Tse, M. Klawonn, C. Dobler, B. Bitterlich, A. Grotevendt, M. Beller, Org. Lett. 2005, 7, 3393;
- 4gK. Ma, J. You, Chem. Eur. J. 2007, 13, 1863;
- 4hT. Arai, T. Mizukami, A. Yanagisawa, Org. Lett. 2007, 9, 1145;
- 4iS. Enthaler, B. Hagemann, S. Bhor, G. Anilkumar, M. K. Tse, B. Bitterlich, K. Junge, G. Erre, M. Beller, Adv. Synth. Catal. 2007, 349, 853; for recent reviews about palladacycles, see:
- 4jI. P. Beletskaya, A. V. Cheprakov, J. Organomet. Chem. 2004, 689, 4055;
- 4kJ. Dupont, C. S. Consorti, J. Spencer, Chem. Rev. 2005, 105, 2527.
- 5
- 5aC. López, A. Caubet, S. Pérez, X. Solans, M. Font-Bardía, E. Molins, Eur. J. Inorg. Chem. 2006, 3974, and references therein;
- 5breview on Pt-pincer complexes: M. Albrecht, G. van Koten, Angew. Chem. 2001, 113, 3866;
10.1002/1521-3757(20011015)113:20<3866::AID-ANGE3866>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 2001, 40, 3750.10.1002/1521-3773(20011015)40:20<3750::AID-ANIE3750>3.0.CO;2-6 CAS PubMed Web of Science® Google Scholar
- 6
- 6aS. Jautze, P. Seiler, R. Peters, Angew. Chem. 2007, 119, 1282;
10.1002/ange.200603568 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1260;
- 6bS. Jautze, P. Seiler, R. Peters, Chem. Eur. J. 2008, 14, 1430;
- 6cS. Jautze, R. Peters, Angew. Chem. 2008, 120, 9424; Angew. Chem. Int. Ed. 2008, 47, 9284.
- 7Cycloplatination of racemic Ugi-amine:
- 7aP. R. R. Ranatunge-Bandarage, B. H. Robinson, J. Simpson, Organometallics 1994, 13, 500.
- 8CCDC 698461 (2) and 698460 (a derivative prepared from 4 a) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 9
- 9aR. K. Thalji, J. A. Ellman, R. G. Bergman, J. Am. Chem. Soc. 2004, 126, 7192;
- 9bS. J. O'Malley, K. L. Tan, A. Watzke, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2005, 127, 13496;
- 9cR. M. Wilson, R. K. Thalji, R. G. Bergman, J. A. Ellman, Org. Lett. 2006, 8, 1745.
- 10
- 10aC. Liu, X. Han, X. Wang, R. A. Widenhoefer, J. Am. Chem. Soc. 2004, 126, 3700;
- 10bX. Han, R. A. Widenhoefer, Org. Lett. 2006, 8, 3801;
- 10cC. Liu, C. F. Bender, X. Han, R. A. Widenhoefer, Chem. Commun. 2007, 3607. Pioneering studies on the use of stoichiometric amounts of PdII in intramolecular Friedel–Crafts-type alkylations of indoles for alkaloid syntheses (desethylibogamine and ibogamine):
- 10dB. M. Trost, J. P. Genêt, J. Am. Chem. Soc. 1976, 98, 8516; and
- 10eB. M. Trost, J. P. Genêt, J. Am. Chem. Soc. 1978, 100, 3930. Catalytic amounts of PdII in related asymmetric Fujiwara–Moritani cyclizations:
- 10fJ. A. Schiffner, A. B. Machotta, M. Oestreich, Synlett 2008, 2271.
- 11J. E. Saxton, Nat. Prod. Rep. 1997, 14, 559.
- 12C. Liu, R. A. Widenhoefer, Org. Lett. 2007, 9, 1935.
- 13Although isomerically pure E-3 e did not react, the 3:1 Z/E isomeric mixture gave a significantly higher yield than 75 % (checked twice). Similarly, a 1:1 mixture gave a yield of 55 %.
- 14Selected applications of previous enantiopure platinacycle complexes: aldol additions:
- 14aF. Gorla, A. Togni, L. M. Venanzi, A. Albinati, F. Lianza, Organometallics 1994, 13, 1607;
- 14bJ. M. Longmire, X. Zhang, M. Shang, Organometallics 1998, 17, 4374;
- 14cY. Motoyama, H. Kawakami, K. Shimozono, K. Aoki, H. Nishiyama, Organometallics 2002, 21, 3408; Diels–Alder reactions:
- 14dW.-C. Yeo, J. J. Vittal, L. L. Koh, G.-K. P. H. Tan, Leung, Organometallics 2004, 23, 3474.