Journal list menu
Export Citations
Download PDFs
Cover Pictures
Cover Picture: Synthesis of Azepine Derivatives by Rhodium-Catalyzed Tandem 2,3-Rearrangement/Heterocyclization (Angew. Chem. Int. Ed. 43/2012)
- Page: 10673
- First Published: 17 September 2012

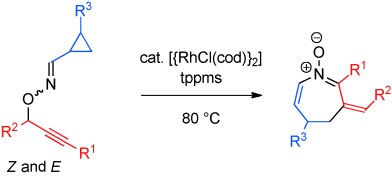
Heterocycle construction through the formation of an azametallacycle from an N-allenylnitrone intermediate is demonstrated in the Communication of I. Nakamura et al. on page 10816 ff. The inherently unstable intermediate can be generated in situ by catalytic 2,3-rearrangement from O-propargylic oximes. This tandem reaction can be compared to a flying trapeze knee hang maneuver.
Inside Cover: Giant Spin-Orbit Effects on NMR Shifts in Diamagnetic Actinide Complexes: Guiding the Search of Uranium(VI) Hydride Complexes in the Correct Spectral Range (Angew. Chem. Int. Ed. 43/2012)
- Page: 10674
- First Published: 04 October 2012

The known 1H NMR chemical shifts of diamagnetic compounds so far range from around δ=−60 ppm to about +20 ppm. In their Communication on page 10884 ff., P. Hrobarik, M. Kaupp, et al. predict that, because of giant relativistic spin-orbit effects, the 1H NMR shifts of the to-date elusive uranium(VI) hydride complexes will be between δ=+40 ppm and more than +150 ppm, a completely unprecedented range. Knowing the correct spectral range to look for should facilitate the characterization of this new compound class.
Inside Back Cover: Polymersome Colloidosomes for Enzyme Catalysis in a Biphasic System (Angew. Chem. Int. Ed. 43/2012)
- Page: 10903
- First Published: 12 October 2012
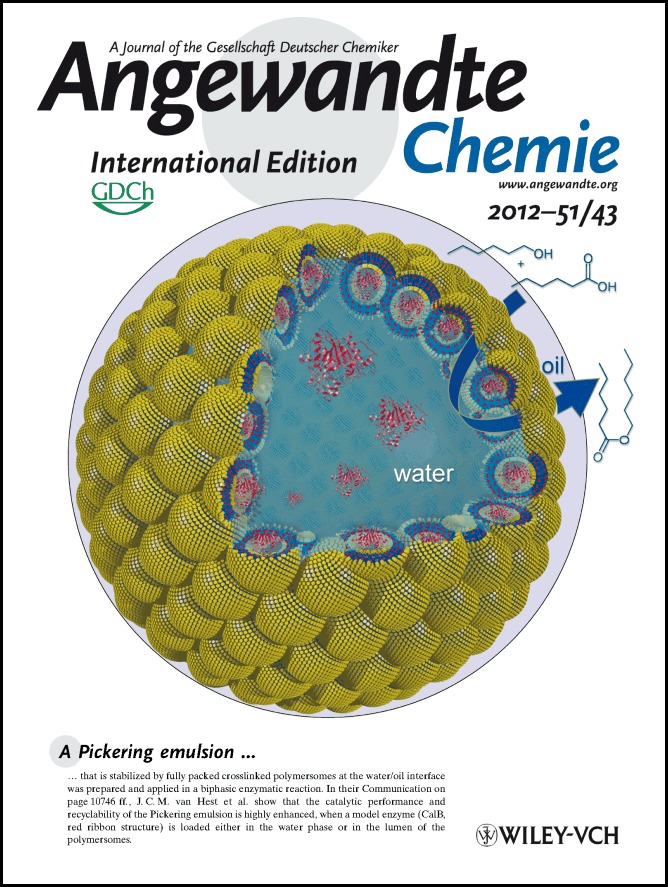
A Pickering emulsion that is stabilized by fully packed crosslinked polymersomes at the water/oil interface was prepared and applied in a biphasic enzymatic reaction. In their Communication on page 10746 ff., J. C. M. van Hest et al. show that the catalytic performance and recyclability of the Pickering emulsion is highly enhanced, when a model enzyme (CalB, red ribbon structure) is loaded either in the water phase or in the lumen of the polymersomes.
Back Cover: A Phenylboronate-Functionalized Polyion Complex Micelle for ATP-Triggered Release of siRNA (Angew. Chem. Int. Ed. 43/2012)
- Page: 10904
- First Published: 12 October 2012

Delivery of small interfering RNA is described by K. Kataoka et al. in their Communication on page 10751 ff. siRNA encapsulated in a phenylboronate-functionalized polyion complex micelle shows specific binding between the pendent phenylboronate and the 3′-ribose moiety of the siRNA, stabilizing the complex under extracellular conditions. This complex is disrupted upon addition of ATP at a concentration comparable to that inside cells.
Editorial
Editorial: Australian Chemistry under the Spotlight
- Pages: 10676-10677
- First Published: 13 July 2012
Graphical Abstract
Graphical Abstract: Angew. Chem. Int. Ed. 43/2012
- Pages: 10679-10692
- First Published: 17 October 2012
Corrigendum
Corrigendum: D-Luciferin Analogues: a Multicolor Toolbox for Bioluminescence Imaging
- Page: 10691
- First Published: 17 October 2012
Addition
Addition: A Sinter-Resistant Catalytic System Based on Platinum Nanoparticles Supported on TiO2 Nanofibers and Covered by Porous Silica
- Page: 10692
- First Published: 17 October 2012
News
Spotlights on our sister journals: Angew. Chem. Int. Ed. 43/2012
- Pages: 10694-10696
- First Published: 17 October 2012
Author Profile
News
Book Review
Highlights
5-Hydroxymethylcytosine
Sequencing the Sixth Base (5-Hydroxymethylcytosine): Selective DNA Oxidation Enables Base-Pair Resolution†
- Pages: 10704-10707
- First Published: 26 September 2012
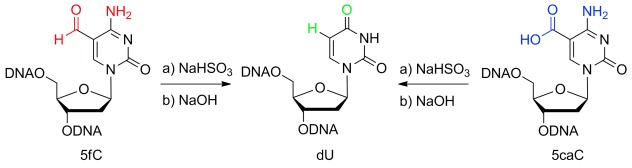
Sodium bisulfite promotes both the deformylative deamination of 5-formylcytosine (5fC) and the decarboxylative deamination of 5-carboxylcytosine (5caC; see picture). By coupling this bisulfite chemistry with selective oxidations of individual DNA bases, new methods allow, for the first time, the sequencing of 5-hydroxymethylcytosine (5hmC) with single-base-pair resolution.
Materials Science
Photostimulated and Photosuppressed Phase Transitions in Liquid Crystals
- Pages: 10708-10710
- First Published: 07 September 2012
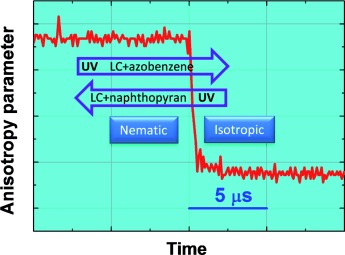
Shape matters: Changes, which are brought about by irradiation, to the properties of a medium have been of immense interest not only in terms of basic science, but also for applications such as data storage media and molecular devices. In the light of a recent publication reporting a photodriven liquid to liquid crystalline transformation (see figure), an overview of similar transitions is presented.
Minireview
CH Bond Activation
Catalytic, Mild, and Selective Oxyfunctionalization of Linear Alkanes: Current Challenges
- Pages: 10712-10723
- First Published: 20 September 2012
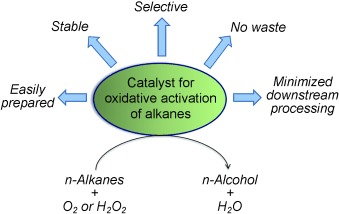
Seeking ideality: Intensive efforts have been invested into the discovery and/or engineering of a range of catalysts, ideally meeting the criteria shown in the picture, for the oxidative CH bond activation of linear alkanes. The comparison between chemical and enzymatic catalysts for alkane oxyfunctionalization is an extremely useful strategy to gain insights into the fundamental mechanisms of this chemistry.
Review
Imaging Agents
Red Fluorescent Proteins: Advanced Imaging Applications and Future Design
- Pages: 10724-10738
- First Published: 31 July 2012
Well red: Modern red fluorescent proteins (RFPs) provide new possibilities to study biological processes at the levels from single molecules to whole organisms (see scheme). Conventional and far-red RFPs, RFPs with a large Stokes shift, fluorescent timers, irreversibly photoactivatable, and reversibly photoswitchable RFPs are discussed in relationship to advanced imaging approaches.
Communications
Gas Sensors
Mechanical Drawing of Gas Sensors on Paper†
- Pages: 10740-10745
- First Published: 04 October 2012

Pencil it in: Mechanical abrasion of compressed single-walled carbon nanotubes (SWCNTs) on the surface of paper produces sensors capable of detecting NH3 gas at sub-ppm concentrations. This method of fabrication is simple, inexpensive, and entirely solvent-free, and avoids difficulties arising from the inherent instability of many SWCNT dispersions.
Biocatalytic Nanoreactors
Polymersome Colloidosomes for Enzyme Catalysis in a Biphasic System†
- Pages: 10746-10750
- First Published: 28 September 2012
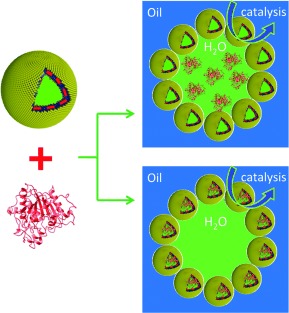
A polymersome-stabilized Pickering emulsion was prepared and applied in a biphasic enzymatic reaction. This type of Pickering emulsion was stabilized by fully packed crosslinked polymersomes at the water/oil interface. CalB, as a model enzyme (red ribbon structure), was loaded either in the water phase or in the lumen of the polymersomes of the Pickering emulsion (see picture), which highly enhanced its catalytic performance and recyclability.
siRNA Delivery
A Phenylboronate-Functionalized Polyion Complex Micelle for ATP-Triggered Release of siRNA†
- Pages: 10751-10755
- First Published: 21 August 2012
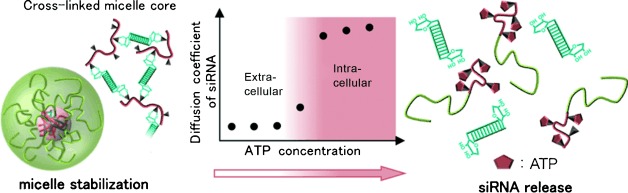
PIC-ing a winner: siRNA encapsulated by a phenylboronate-functionalized polyion complex (PIC) micelle shows binding between the phenylboronate and the 3′ ribose of the siRNA (see scheme), stabilizing the complex under conditions equivalent to the extracellular environment. This complex is disrupted in response to addition of ATP, at a concentration comparable to that inside cells.
Solid-State Protein NMR Spectroscopy
Backbone Assignment of Fully Protonated Solid Proteins by 1H Detection and Ultrafast Magic-Angle-Spinning NMR Spectroscopy†
- Pages: 10756-10759
- First Published: 28 September 2012
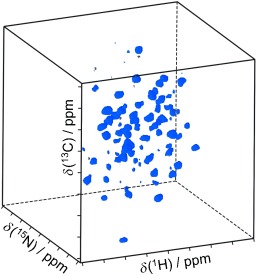
Narrow 1H NMR linewidths can be obtained for fully protonated protein samples in the solid state by using ultrafast magic-angle spinning (60 kHz). Medium-size microcrystalline and noncrystalline proteins can be analyzed without any need for deuteration of the protein sample. This approach provides assignments of the backbone 1H, 15N, 13Cα, and 13CO resonances and yields information about 1H–1H proximities.
Water Splitting
p-Type InP Nanopillar Photocathodes for Efficient Solar-Driven Hydrogen Production†
- Pages: 10760-10764
- First Published: 23 September 2012
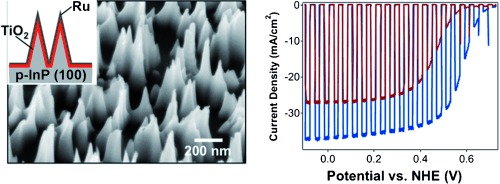
Perfect texture: The roles of surface nanotexturing, TiO2 passivation, and a ruthenium cocatalyst on the photoelectrochemical evolution of hydrogen by using p-InP photocathodes are investigated. Higher current densities and more favorable onset potentials are observed after surface nanotexturing. NHE=normal hydrogen electrode.
Microfluidic Bioassay
Programmable Magnetic Tweezers and Droplet Microfluidic Device for High-Throughput Nanoliter Multi-Step Assays†
- Pages: 10765-10769
- First Published: 26 September 2012
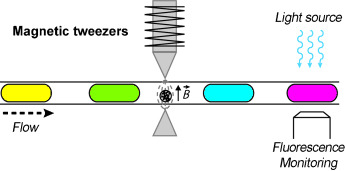
Tweezing out the answer: A microfluidic device combining droplets (less than 100 nL) and magnetic particles (see scheme) was implemented for fast heterogeneous multiplexed assays. Magnetic tweezers can perform the manipulations required in an immunoassay (capture, extraction, mixing, and rinsing). This method was applied to the diagnosis of congenital hypothyroidism with 14 pM sensitivity.
Functional Materials
Perovskite B-Site Compositional Control of [110]p Polar Displacement Coupling in an Ambient-Pressure-Stable Bismuth-based Ferroelectric†
- Pages: 10770-10775
- First Published: 28 September 2012
![Perovskite B-Site Compositional Control of [110]p Polar Displacement Coupling in an Ambient-Pressure-Stable Bismuth-based Ferroelectric](/cms/asset/5cace369-1e26-4c9a-ad8e-05de288ef6f4/mcontent.jpg)
Off the axis: A new lead-free bismuth based perovskite has been formed at ambient pressure in the polar Pmc21 structure. Measurements give evidence for ferroelectricity and piezoelectricity. The material is significant due to a rotation of the polarization direction off the [111]p axis, making it important to the design of materials with a morphotropic phase boundary.
Nanoparticles Self-Assembly
PbS–Organic Mesocrystals: The Relationship between Nanocrystal Orientation and Superlattice Array†
- Pages: 10776-10781
- First Published: 25 September 2012
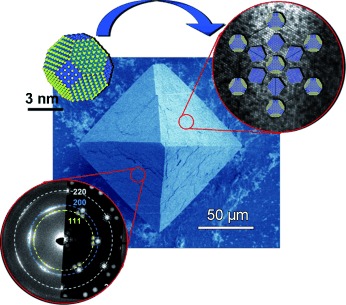
Try to make it ordered! Micrometer-sized PbS–organic mesocrystals show a long-range order of nanoparticles within an fcc superlattice combined with preferred orientational ordering of truncated octahedrally shaped PbS cores (see picture). The concept of formation and structuring of mesocrystalline materials is perfectly illustrated this system.
Photoluminescence
Piezochromic Luminescence Based on the Molecular Aggregation of 9,10-Bis((E)-2-(pyrid-2-yl)vinyl)anthracene†
- Pages: 10782-10785
- First Published: 28 September 2012
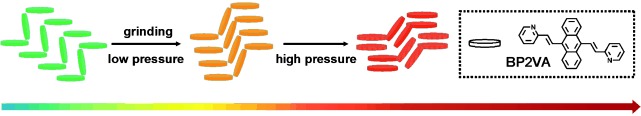
A chameleon under pressure: The observed piezochromic behavior of the title compound (BP2VA) was found to depend on its molecular aggregation state and specifically on the strength of the π–π interaction between the anthracene rings of adjacent molecules. When BP2VA is ground or placed under pressure, its molecular aggregation state changes, and a red shift in the fluorescence emission from green via orange to red occurs (see picture).
Electrocatalysis
Hydroxide Ion Conducting Antimony(V)-Doped Tin Pyrophosphate Electrolyte for Intermediate-Temperature Alkaline Fuel Cells†
- Pages: 10786-10790
- First Published: 26 September 2012
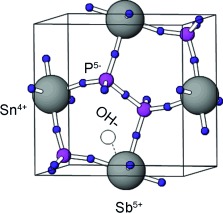
Ion conductor: A series of Sn1–xAxP2O7 (AV=V, Nb, Ta, and Sb) compounds was synthesized, among which Sn0.92Sb0.08P2O7 (see picture) showed the highest hydroxide ion conductivity in the temperature range of 50–200 °C (0.08 S cm−1 at 100 °C and 0.05 S cm−1 at 200 °C). This high conductivity was also confirmed under fuel-cell-operating conditions.
Materials Synthesis
“Hard–Soft” Synthesis of SrCrO3−δ Superstructure Phases†
- Pages: 10791-10794
- First Published: 26 September 2012
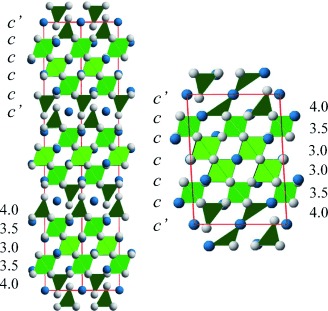
Dense metastable phases obtained under “hard” high-pressure conditions may contain instabilities such as unusual oxidation states or coordination environments that may be partially relieved by “soft” low-temperature chemistry. The synthesis of SrCrO2.8 (see picture, left) and SrCrO2.75 (right) phases from the high-pressure perovskite SrCrO3 leads to a relaxation of the coordination around Cr4+ from octahedral to tetrahedral.
Live-Cell Imaging
Monitoring β-Secretase Activity in Living Cells with a Membrane-Anchored FRET Probe†
- Pages: 10795-10799
- First Published: 28 September 2012
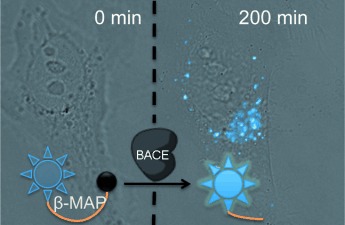
Turn it on! β-MAP is a sensitive FRET probe with specificity for monitoring the enzyme β-secretase (BACE), which is associated with Alzheimer's disease. After hydrolysis by the enzyme BACE, the probe fluoresces and thus allows real-time spatial and temporal assessment of enzymatic activity in living cells. β-MAP was used to confirm the cellular efficacy of a reported inhibitor without the need for mutated cell lines or antibodies.
Protein Stabilization
Self-Healing Microencapsulation of Biomacromolecules without Organic Solvents†
- Pages: 10800-10803
- First Published: 26 September 2012
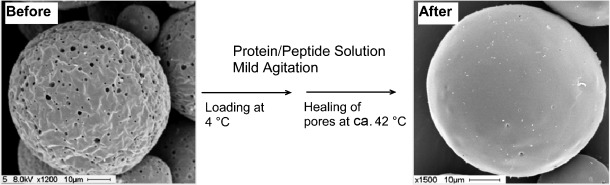
Capture and seal off all exits! Biomacromolecules are routinely microcapsulated in poly(lactic-co-glycolic acid) (PLGA) in multiple complex steps that are deleterious to the biomacromolecule. In contrast, PLGA encapsulation based on self-healing (see picture) shows high efficiency without protein damage and enables the stabilization and slow release of proteins.
Synthetic Methods
Photo-fluorodecarboxylation of 2-Aryloxy and 2-Aryl Carboxylic Acids†
- Pages: 10804-10807
- First Published: 28 September 2012
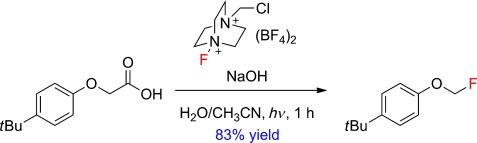
Coming to light: The title reaction simply requires an aqueous alkaline solution of Selectfluor and light. The method is inexpensive and effective for a wide range of neutral and electron-poor 2-aryloxy and 2-aryl acetic acids to provide fluoromethyl ethers (see scheme) and benzyl fluorides, respectively. The mechanism most likely proceeds through an initial aryl excitation with a subsequent single-electron transfer.
CC Coupling
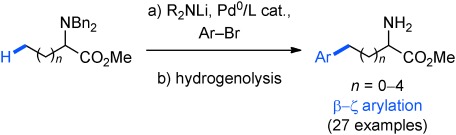
Synthesis of Aromatic α-Aminoesters: Palladium-Catalyzed Long-Range Arylation of Primary C H Bonds†
H Bonds†
- Pages: 10808-10811
- First Published: 28 September 2012
Organometallic Catalysis
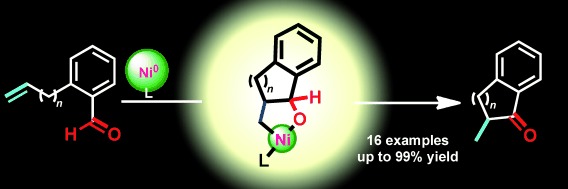
Synthesis of Five- and Six-Membered Benzocyclic Ketones through Intramolecular Alkene Hydroacylation Catalyzed by Nickel(0)/N-Heterocyclic Carbenes†
- Pages: 10812-10815
- First Published: 25 September 2012
Heterocycles
Synthesis of Azepine Derivatives by Rhodium-Catalyzed Tandem 2,3-Rearrangement/Heterocyclization†
- Pages: 10816-10819
- First Published: 31 August 2012
Natural Products
Strophasterols A to D with an Unprecedented Steroid Skeleton: From the Mushroom Stropharia rugosoannulata†
- Pages: 10820-10822
- First Published: 26 September 2012
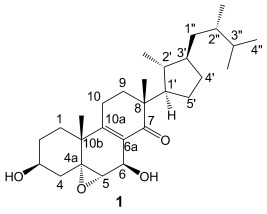
Skeletons in the closet: Four new compounds have been isolated from the title mushroom. The compounds display a new steroid skeleton (e.g., 1) not previously reported for steroids. Preliminary bioactivity tests show that compound 1 can protect neuronal cells by attenuating the endoplasmic reticulum stress, and has weak anti-methicillin-resistant Staphylococcus aureus activity.
Dehydrogenative Coupling
Iron-Facilitated Oxidative Dehydrogenative CO Bond Formation by Propargylic C H Functionalization†
H Functionalization†
- Pages: 10823-10826
- First Published: 09 October 2012
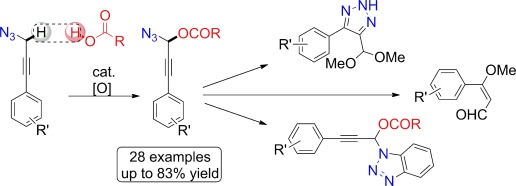
Hot couple: Propargyl azides were coupled with carboxylic acids by an iron-catalyzed dehydrogenative CO bond formation (see scheme). This method enables propargylic C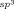 H functionalization under mild reaction conditions and also may involve the application of the azido moiety as an assisting group in CH activation.
H functionalization under mild reaction conditions and also may involve the application of the azido moiety as an assisting group in CH activation.
Domino Reactions
Enantioselective Copper(I)-Catalyzed Borylative Aldol Cyclizations of Enone Diones†
- Pages: 10827-10831
- First Published: 28 September 2012
Piña colato? In the presence of a chiral CuI/bisphosphine complex and B2(pin)2, enone diones undergo diastereo- and enantioselective desymmetrization to deliver highly functionalized bicyclic products. The products can be used as substrates in additional transformations. pin=pinacolato, Cy=cyclohexyl.
NHC Complexes
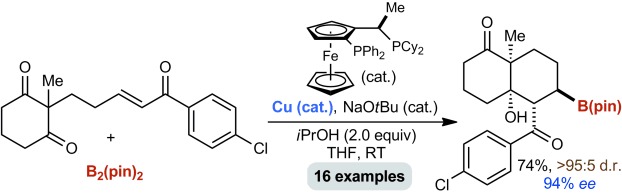
Transition Metal Complexes of Anionic N-Heterocyclic Dicarbene Ligands†
- Pages: 10832-10835
- First Published: 07 September 2012
A tale of two carbenes: Reaction of :C[N(2,6-iPr2C6H3)CH]2 (IPr) with Mn3(mes)6 (mes=2,4,6-trimethylphenyl) yielded the trigonal planar complex [Mn(IPr)(mes)2]. Reduction of this species with potassium/graphite in THF afforded the polymeric dicarbene-bridged species K[{:C[N(2,6-iPr2C6H3)]2(CH)C}2Mn(mes)(thf)]⋅THF (see picture). The anionic moiety in this complex is the first reported example of a transition metal complex containing an N-heterocyclic dicarbene ligand. Gray C, blue N, red Mn.
Phosphorus Cations
Structure Elucidation
Unconventional Reactivity of Imidazolylidene Pyridylidene Ligands in Iridium(III) and Rhodium(III) Complexes†
- Pages: 10841-10845
- First Published: 26 September 2012
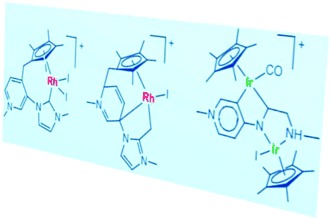
Expect the unexpected: The reactions of a series of imidazolium pyridinium salts with [{IrCp*Cl2}2] and [{RhCp*Cl2}2] afford a series of complexes. Together with the expected bis(NHC) complexes, some species resulting from CC coupling between the pyridylidene and Cp* ligands were observed (see figure; Cp*=pentamethylcyclopentadienyl).
Natural Products
Total Synthesis of the Nominal Didemnaketal A†
- Pages: 10846-10850
- First Published: 26 September 2012
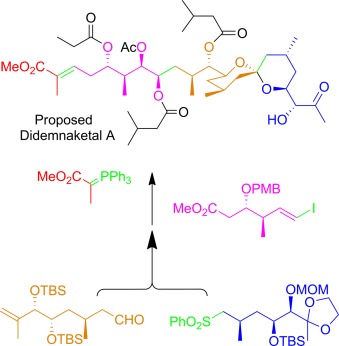
False identity: The synthesis of a natural product described by Faulkner and co-workers two decades ago has revealed the need for the revision of some stereochemical assignments. The key steps in this flexible route, which could provide access to stereodefined analogues for biological evaluation, included a Julia coupling, a Suzuki–Miyaura reaction, and Wittig olefination (see scheme; MOM, PMB, and TBS are protecting groups).
Synthetic Methodology
Characterization and Utility of N-Unsubstituted Imines Synthesized from Alkyl Azides by Ruthenium Catalysis†
- Pages: 10851-10855
- First Published: 28 September 2012
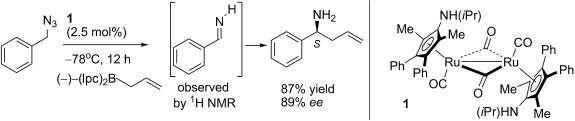
Shine a light: A fluorescent light-induced synthetic method for the title compounds has been developed and the chemoselective nature of the reaction is highlighted by the observation of the cis/trans isomers of various N-unsubstituted imines. The synthetic utility of this method is demonstrated by the one-pot imine formation/asymmetric allylation sequence of benzyl azide catalyzed by 1. (Ipc=isopinocampheyl).
Asymmetric Synthesis
N-(Diazoacetyl)oxazolidin-2-thiones as Sulfur-Donor Reagents: Asymmetric Synthesis of Thiiranes from Aldehydes†
- Pages: 10856-10860
- First Published: 26 September 2012
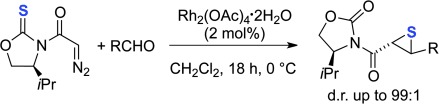
Sulfur tyranny: Thiiranes, instead of oxiranes, can be obtained in a highly stereoselective manner through the cycloaddition reaction of N-acyl oxazolidine tethered diazo thione compounds with aldehydes catalyzed by RhII. Thus, this reaction provides versatile adducts S functionalized at both the α and β position, with concomitant generation of two contiguous stereocenters.
Synthetic Methods
An Efficient Route to Polysubstituted Tetrahydronaphthols: Silver-Catalyzed [4+2] Cyclization of 2-Alkylbenzaldehydes and Alkenes†
- Pages: 10861-10865
- First Published: 26 September 2012
![An Efficient Route to Polysubstituted Tetrahydronaphthols: Silver-Catalyzed [4+2] Cyclization of 2-Alkylbenzaldehydes and Alkenes](/cms/asset/e9290035-ae9c-44b8-9e3f-f7657eec6d10/mcontent.jpg)
Silver bullet: A methodology for stereoselective synthesis of polysubstituted tetrahydronaphthols catalyzed by [Ag+]/NPO has been developed. The reactions proceeded through an unprecedented [4+2] cyclization of 2-(2-formylphenyl)ethanone and an alkene, in both inter- and intramolecular fashion. NPO=pyridine N-oxide.
Homogeneous Catalysis
The Thiolate-Catalyzed Intermolecular Crossed Tishchenko Reaction: Highly Chemoselective Coupling of Two Different Aromatic Aldehydes†
- Pages: 10866-10870
- First Published: 26 September 2012
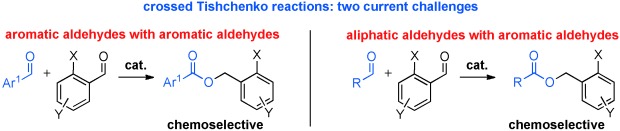
Crossed products: Ortho-substituted benzaldehydes react with other aromatic aldehydes in a highly selective, atom-economical Tishchenko disproportionation (see scheme) in the presence of a readily prepared, inexpensive thiolate-based catalyst. The methodology is of exceptionally wide scope and exhibits a high functional-group tolerance.
Dicarbaporphyrinoids
Two-Step Synthesis of Stable Dioxadicarbaporphyrins from Bis(3-indenyl)methane†
- Pages: 10871-10875
- First Published: 23 September 2012
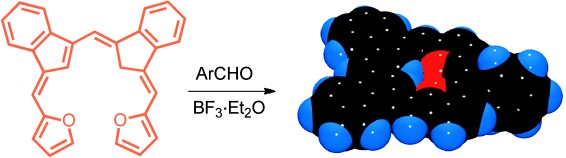
Porphyrin without N: Bilin analogues and related aromatic dicarbaporphyrinoids have been prepared from bis(3-indenyl)methane. Even though all four pyrrole rings from the porphyrin macrocycle have been replaced by two furan and two indene subunits, the system retains porphyrin-like UV/Vis spectra and highly diatropic characteristics.
Asymmetric Catalysis
Enantioselective Rhodium-Catalyzed Synthesis of Branched Allylic Amines by Intermolecular Hydroamination of Terminal Allenes†
- Pages: 10876-10879
- First Published: 26 September 2012
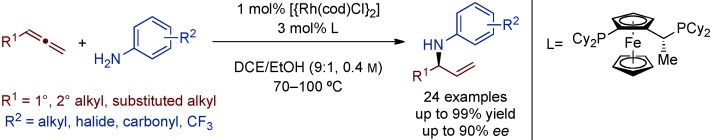
Branching out: The rhodium-catalyzed enantioselective hydroamination of monosubstituted allenes with anilines permits the atom-economic synthesis of valuable branched allylic amines. In contrast to previous linear selective allene hydroaminations, a RhI/Josiphos catalyst system (see scheme; cod=1,5-cyclooctadiene, DCE=1,2-dichloroethane) allows branched allylic amines to be obtained with perfect regioselectivity, high yield, and good enantioselectivity.
B,N Heterocycles
Photoisomerization of 1,2-Dihydro-1,2-Azaborine: A Matrix Isolation Study†
- Pages: 10880-10883
- First Published: 28 September 2012
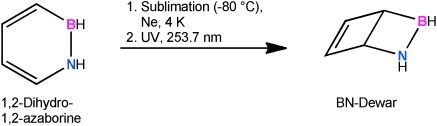
Closing the loop: Photoisomerization of 1,2-dihydro-1,2-azaborine in neon, argon, or xenon at 4 K with UV light (253.7 nm) as part of a matrix isolation study led to the Dewar form as the only photoproduct, in agreement with the vibrational spectra computed for possible isomers of 1,2-dihydro-1,2-azaborine.
Actinide Complexes
Giant Spin-Orbit Effects on NMR Shifts in Diamagnetic Actinide Complexes: Guiding the Search of Uranium(VI) Hydride Complexes in the Correct Spectral Range†
- Pages: 10884-10888
- First Published: 28 September 2012
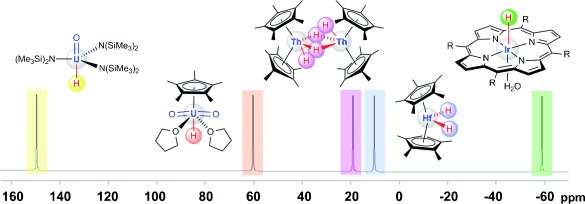
Looking in the right (NMR) ballpark: The 13C shifts of carbon atoms σ-bonded to uranium(VI) centers, and in particular the 1H shifts of UVI bound hydride ligands, are predicted to be at unprecedentedly high frequencies (see picture), as a result of unexpectedly large spin-orbit effects. Based on relativistic quantum-chemical calculations, the right spectral ranges are suggested, which may allow identification of such compounds.
Natural Product Synthesis
Enantioselective Total Synthesis of the Diterpene (+)-Cubitene†
- Pages: 10889-10892
- First Published: 25 September 2012
From termite soldier's secretions: The enantioselective total synthesis of the diterpene (+)-cubitene is described. The route is characterized by the cyclization of a carvone-derived C20 allylphosphate with SmI2, followed by fragmentation to the twelve-membered ring. As a result, perfect stereocontrol of the isopropenyl-substituted positions is achieved.
Methanetrisamidines
Synthesis, Structure, Tautomerism, and Reactivity of Methanetrisamidines†
- Pages: 10893-10897
- First Published: 20 September 2012
Porphyrin Metalation
Activation Energy for the Self-Metalation Reaction of 2H-Tetraphenylporphyrin on Cu(111)†
- Pages: 10898-10901
- First Published: 26 September 2012
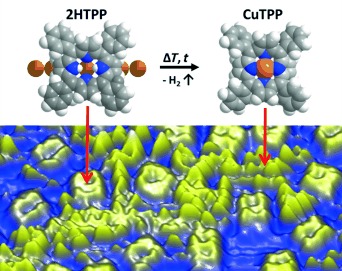
By simply counting individual molecules in STM images after defined heating steps, the kinetic parameters and the activation energy of a complex surface reaction can be determined quantitatively. This procedure was demonstrated for the metalation of 2H-tetraphenylporphyrin (2HTPP) with substrate atoms on a Cu(111) surface.