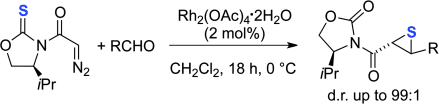N-(Diazoacetyl)oxazolidin-2-thiones as Sulfur-Donor Reagents: Asymmetric Synthesis of Thiiranes from Aldehydes†
Dr. Israel Cano
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorDr. Enrique Gómez-Bengoa
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorDr. Aitor Landa
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorDr. Miguel Maestro
Departamento de Química Fundamental, Facultad de Ciencias, Universidade da Coruña, Campus Zapateira s/n, 15071A Coruña (Spain)
X-Ray analyses.
Search for more papers by this authorDr. Antonia Mielgo
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorIurre Olaizola
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorProf. Dr. Mikel Oiarbide
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorCorresponding Author
Prof. Dr. Claudio Palomo
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)Search for more papers by this authorDr. Israel Cano
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorDr. Enrique Gómez-Bengoa
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorDr. Aitor Landa
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorDr. Miguel Maestro
Departamento de Química Fundamental, Facultad de Ciencias, Universidade da Coruña, Campus Zapateira s/n, 15071A Coruña (Spain)
X-Ray analyses.
Search for more papers by this authorDr. Antonia Mielgo
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorIurre Olaizola
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorProf. Dr. Mikel Oiarbide
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Search for more papers by this authorCorresponding Author
Prof. Dr. Claudio Palomo
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)
Departamento de Química Orgánica I, Universidad del País Vasco, Manuel Lardizabal 3, 20018 San Sebastián (Spain)Search for more papers by this authorFinancial support was provided by the University of the Basque Country UPV/EHU (UFI 11/22), Basque Government (GV grant No IT-291-07), and Ministerio de Ciencia e Innovación (MICINN, Grant CTQ2007-68095-C02), Spain. A.L. thanks MICINN and the European Social Foundation for a Ramón y Cajal contract. I.O. thanks MCINN for a fellowship. We also thank SGIker (UPV/EHU) for providing NMR, HRMS, X-ray, and computational resources.
Graphical Abstract
Sulfur tyranny: Thiiranes, instead of oxiranes, can be obtained in a highly stereoselective manner through the cycloaddition reaction of N-acyl oxazolidine tethered diazo thione compounds with aldehydes catalyzed by RhII. Thus, this reaction provides versatile adducts S functionalized at both the α and β position, with concomitant generation of two contiguous stereocenters.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201204771_sm_miscellaneous_information.pdf3.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Organosulfur Chemistry in Asymmetric Synthesis (Eds.: ), Wiley-VCH, Weinheim, 2008;
10.1002/9783527623235 Google Scholar
- 1bM. Mellah, A. Voituriez, E. Schulz, Chem. Rev. 2007, 107, 5133–5209.
- 2
- 2aD. J. Procter, J. Chem. Soc. Perkin Trans. 1 2001, 335–354; for asymmetric synthesis of tertiary thiols and thioethers, see:
- 2bJ. Clayden, P. MacLellan, Beilstein J. Org. Chem. 2011, 7, 582–595; for metal-catalyzed CS bond formation, see:
- 2cT. Kondo, T.-a. Mitsudo, Chem. Rev. 2000, 100, 3205–3220;
- 2dW. Adam, R. M. Bargon, Chem. Rev. 2004, 104, 251–261;
- 2eY. Zhang, J. Wang, Coord. Chem. Rev. 2010, 254, 941–953.
- 3Leading examples of asymmetric electrophilic α-sulfenylations: Stoichiometric:
- 3aD. A. Evans, K. R. Campos, J. S. Tedrow, F. E. Michael, M. R. Gagné, J. Am. Chem. Soc. 2000, 122, 7905–7920;
- 3bD. Enders, A. Schaadt, Synlett 2002, 498–500: organocatalytic:
- 3cM. Marigo, T. C. Wabnitz, D. Fielenbach, K. A. Jørgensen, Angew. Chem. 2005, 117, 804–807;
10.1002/ange.200462101 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 794–797;
- 3dG. L. Zhao, R. Rios, J. Vesely, L. Eriksson, A. Cordova, Angew. Chem. 2008, 120, 8596–8600; Angew. Chem. Int. Ed. 2008, 47, 8468–8472;
- 3eL. Fang, A. Lin, H. Hu, C. Zhu, Chem. Eur. J. 2009, 15, 7039–7043; metal catalyzed:
- 3fM. Jereb, A. Togni, Chem. Eur. J. 2007, 13, 9384–9392.
- 4For a review on asymmetric sulfa-Michael additions, see: D. Enders, K. Lüttgen, A. A. Narine, Synthesis 2007, 959–980.
- 5For a general review on thiiranes, see: “Thiiranes and Derivatives” M. Saito, J. Nakayama in Science of Syntheses, Houben-Weyl Methods of Molecular Transformations, Vol. 39 (Eds.: ), Thieme, Stuttgart, 2008, pp. 589–658 and Ref. [2d].
- 6Asymmetric synthesis of thiiranes from chiral nonracemic precursors: allenes:
- 6aC. Zhou, C. Fu, S. Ma, Angew. Chem. 2007, 119, 4457–4459; Angew. Chem. Int. Ed. 2007, 46, 4379–4381; oxiranes:
- 6bM. Lee, M. M. Bernardo, S. O. Meroueh, S. Brown, R. Fridman, S. Mobashery, Org. Lett. 2005, 7, 4463–4465; β-thiocyanoalcohols:
- 6cE. Łukowska, J. Plenkiewicz, Tetrahedron: Asymmetry 2007, 18, 1202–1209; synthesis of chiral nonracemic thiiranium ions:
- 6dS. E. Denmark, T. Vogler, Chem. Eur. J. 2009, 15, 11737–11745.
- 7
- 7aC. Palomo, M. Oiarbide, F. Dias, A. Ortiz, A. Linden, J. Am. Chem. Soc. 2001, 123, 5602–5603;
- 7bC. Palomo, M. Oiarbide, F. Dias, R. López, A. Linden, Angew. Chem. 2004, 116, 3369–3372;
10.1002/ange.200453889 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3307–3310;
- 7cC. Palomo, M. Oiarbide, R. López, P. B. González, E. Gómez-Bengoa, J. M. Saá, A. Linden, J. Am. Chem. Soc. 2006, 128, 15236–15247.
- 8For CS bond formation mediated by sulfur ylides derived from metal carbene, see Ref. [2e]. For metal-catalyzed reactions of α-diazocarbonyl compounds, see:
- 8aM. P. Doyle, M. A. McKervey, T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley-Interscience, New York, 1998;
- 8bZ. Zhang, J. Wang, Tetrahedron 2008, 64, 6577–6605;
- 8cM. P. Doyle, R. Duffy, M. Ratnikov, L. Zhou, Chem. Rev. 2010, 110, 704–724;
- 8dH. M. L. Davies, J. R. Deton, Chem. Soc. Rev. 2009, 38, 3061–3071; for the generation of thiocarbonyl ylides, see:
- 8eA. Padwa, Chem. Soc. Rev. 2009, 38, 3072–3081.
- 9For a review, see:
- 9aV. K. Aggarwal, M. Crimmin, S. Riches in Science of Synthesis, Vol. 37 (Ed.: ), Thieme, Stuttgart, 2008, pp. 321–406; for leading recent examples, see:
- 9bV. K. Aggarwal, J. P. H. Charmant, D. Fuentes, J. N. Harvey, G. Hynd, D. Ohara, W. Picoul, R. L. Robiette, C. Smith, J.-L. Vasse, C. L. Winn, J. Am. Chem. Soc. 2006, 128, 2105–2114 and references therein. For a mechanistic investigation, see:
- 9cD. R. Edwards, P. Montoya-Peleaz, C. M. Crudden, Org. Lett. 2007, 9, 5481–5484.
- 10
- 10aM. P. Doyle, W. Hu, D. J. Timmons, Org. Lett. 2001, 3, 933–935;
- 10bW.-J. Liu, B.-D. Lv, L.-Z. Gong, Angew. Chem. 2009, 121, 6625–6628; Angew. Chem. Int. Ed. 2009, 48, 6503–6506.
- 11See the Supporting Information for details.
- 12N. Iranpoor, H. Firouzabadi, A. A. Jafari, Synth. Commun. 2003, 33, 2321–2327.
- 13For generation, and subsequent cycloaddition, of thiocarbonyl ylides from the RhII-catalyzed cyclization of diazothiocarbonyl compounds, see: Ref. [8e] and
- 13aK. T. Potts, E. Houghton, U. P. Singh, J. Org. Chem. 1974, 39, 3627–3631;
- 13bA. Padwa, F. R. Kinder, L. Zhi, Synlett 1991, 287–288;
- 13cA. Padwa, A. C. Flick, H. I. Lee, Org. Lett. 2005, 7, 2925–2928.
- 14It has been reported that the treatment of a diazothioamide compound related to 1 with rhodium(II) acetate yielded an stable aromatic mesoionic system: K. T. Potts, P. Murphy, J. Chem. Soc. Chem. Commun. 1984, 1348–1349.
- 15An alternative pathway to this tricyclic adduct would involve attack of the thione group onto the epoxide initially generated from the reaction between the aldehyde and the diazocarbonyl moiety. However, the fact that no traces of epoxide product were detected in the reaction mixtures and a 14.4 kcal mol−1 energy barrier is calculated for such epoxide formation (see Ref. [11]), disfavors the epoxide pathway.