Iron-Facilitated Oxidative Dehydrogenative CO Bond Formation by Propargylic C H Functionalization†
H Functionalization†
Teng Wang
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China) http://sklnbd.bjmu.edu.cn/nj
Department of Chemistry, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorWang Zhou
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China) http://sklnbd.bjmu.edu.cn/nj
Search for more papers by this authorHang Yin
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China) http://sklnbd.bjmu.edu.cn/nj
Search for more papers by this authorDr. Jun-An Ma
Department of Chemistry, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorCorresponding Author
Dr. Ning Jiao
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China) http://sklnbd.bjmu.edu.cn/nj
State Key Laboratory of Organometallic Chemistry, Chinese Academy of Sciences, Shanghai 200032 (China)
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China) http://sklnbd.bjmu.edu.cn/njSearch for more papers by this authorTeng Wang
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China) http://sklnbd.bjmu.edu.cn/nj
Department of Chemistry, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorWang Zhou
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China) http://sklnbd.bjmu.edu.cn/nj
Search for more papers by this authorHang Yin
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China) http://sklnbd.bjmu.edu.cn/nj
Search for more papers by this authorDr. Jun-An Ma
Department of Chemistry, Tianjin University, Tianjin 300072 (China)
Search for more papers by this authorCorresponding Author
Dr. Ning Jiao
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China) http://sklnbd.bjmu.edu.cn/nj
State Key Laboratory of Organometallic Chemistry, Chinese Academy of Sciences, Shanghai 200032 (China)
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Xue Yuan Rd. 38, Beijing 100191 (China) http://sklnbd.bjmu.edu.cn/njSearch for more papers by this authorFinancial support from the National Basic Research Program of China (973 Program 2009CB825300), the National Science Foundation of China (No. 21172006), and Peking University are greatly appreciated. We thank Zejun Xu in this group for reproducing the results of 3 ac, 3 ib, and 6 e.
Graphical Abstract
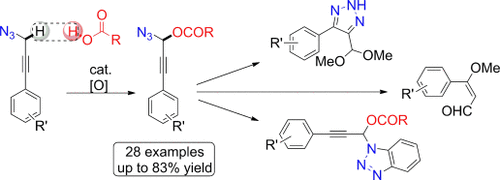
Hot couple: Propargyl azides were coupled with carboxylic acids by an iron-catalyzed dehydrogenative CO bond formation (see scheme). This method enables propargylic C H functionalization under mild reaction conditions and also may involve the application of the azido moiety as an assisting group in CH activation.
H functionalization under mild reaction conditions and also may involve the application of the azido moiety as an assisting group in CH activation.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201205779_sm_miscellaneous_information.pdf1.3 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. V. Ley, A. W. Thomas, Angew. Chem. 2003, 115, 5558;
10.1002/ange.200300594 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 5400.
- 2
- 2aS. B. Singh, G. R. Pettit, J. Org. Chem. 1990, 55, 2797;
- 2bD. Sames, R. Polt, J. Org. Chem. 1994, 59, 4596;
- 2cP. Cristau, J. Vors, J. Zhu, Tetrahedron 2003, 59, 7859;
- 2dB. Schlummer, U. Scholz, Adv. Synth. Catal. 2004, 346, 1599;
- 2eR. Jasti, J. Vitale, S. D. Rychnovsky, J. Am. Chem. Soc. 2004, 126, 9904;
- 2fS. Chamberland, K. A. Woerpel, Org. Lett. 2004, 6, 4739;
- 2gI. D. Gridnev, S. Kikuchi, A. S. Touchy, I. Kadota, Y. Yamamoto, J. Org. Chem. 2007, 72, 8371.
- 3For some reviews, see:
- 3aJ. F. Hartwig, Angew. Chem. 1998, 110, 2154;
10.1002/(SICI)1521-3757(19980803)110:15<2154::AID-ANGE2154>3.0.CO;2-C Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2046;10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 3bA. R. Muci, S. L. Buchwald, Top. Curr. Chem. 2002, 219, 131;
- 3cK. Kunz, U. Scholz, D. Ganzer, Synlett 2003, 2428.
- 4For reviews of cross-dehydrogenative coupling (CDC), see:
- 4aC.-J. Li, Z. Li, Pure Appl. Chem. 2006, 78, 935;
- 4bC.-J. Li, Acc. Chem. Res. 2009, 42, 335;
- 4cC. J. Scheuermann, Chem. Asian J. 2010, 5, 436;
- 4dC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215;
- 4eC. Liu, H. Zhang, W. Shi, A. Lei, Chem. Rev. 2011, 111, 1780.
- 5For CO bond formation through C
 H functionalization utilizing carboxylic acids as nucleophiles, see:
H functionalization utilizing carboxylic acids as nucleophiles, see:
- 5aL. V. Desai, H. A. Malik, M. S. Sanford, Org. Lett. 2006, 8, 1141;
- 5bX. Chen, X.-S. Hao, C. E. Goodhue, J.-Q. Yu, J. Am. Chem. Soc. 2006, 128, 6790;
- 5cG.-W. Wang, T.-T. Yuan, X.-L. Wu, J. Org. Chem. 2008, 73, 4717;
- 5dZ. Ye, W. Wang, F. Luo, S. Zhang, J. Cheng, Org. Lett. 2009, 11, 3974.
- 6For palladium-catalyzed CO bond formation through allylic C
 H functionalization utilizing carboxylic acids as nucleophiles, see:
H functionalization utilizing carboxylic acids as nucleophiles, see:
- 6aA. Heumann, B. Åkermark, S. Hansson, T. Rein, Org. Synth. 1990, 68, 109;
- 6bB. Åkermark, E. M. Larsson, J. D. Oslob, J. Org. Chem. 1994, 59, 5729;
- 6cM. S. Chen, M. C. White, J. Am. Chem. Soc. 2004, 126, 1346;
- 6dM. S. Chen, N, Prabagaran, N. A. Labenz, M. C. White, J. Am. Chem. Soc. 2005, 127, 6970;
- 6eJ. H. Delcamp, M. C. White, J. Am. Chem. Soc. 2006, 128, 15076;
- 6fD. J. Covell, M. C. White, Angew. Chem. 2008, 120, 6548;
10.1002/ange.200802106 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 6448.
- 7
- 7aW. Zhou, L. Zhang, N. Jiao, Angew. Chem. 2009, 121, 7228; Angew. Chem. Int. Ed. 2009, 48, 7094;
- 7bW. Zhou, J. Xu, L. Zhang, N. Jiao, Org. Lett. 2010, 12, 2888.
- 8
- 8aC. Bolm, J. Legros, J. L. Paih, L. Zani, Chem. Rev. 2004, 104, 6217;
- 8bB. D. Sherry, A. Fürstner, Acc. Chem. Res. 2008, 41, 1500;
- 8cA. A. O. Sarhan, C. Bolm, Chem. Soc. Rev. 2009, 38, 2730;
- 8dS. Enthaler, K. Junge, M. Beller, Angew. Chem. 2008, 120, 3363;
10.1002/ange.200800012 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3317;
- 8eE. Nakamura, N. Yoshikai, J. Org. Chem. 2010, 75, 6061;
- 8fC. Sun, B. Li, Z. Shi, Chem. Rev. 2011, 111, 1293.
- 9For some recent examples, see:
- 9aM. S. Chen, M. C. White, Science 2007, 318, 783;
- 9bA. Company, L. Gómez, M. Güell, X. Ribas, J. M. Luis, L. Que, Jr., M. Costas, J. Am. Chem. Soc. 2007, 129, 15766;
- 9cZ. Wang, Y. Zhang, H. Fu, Y. Jiang, Y. Zhao, Org. Lett. 2008, 10, 1863;
- 9dS. Pan, J. Liu, H. Li, Z. Wang, X. Guo, Z. Li, Org. Lett. 2010, 12, 1932;
- 9eC. Qin, N. Jiao, J. Am. Chem. Soc. 2010, 132, 15893;
- 9fM. S. Chen, M. C. White, Science 2010, 327, 566;
- 9gC. Qin, W. Zhou, F. Chen, Y. Ou, N. Jiao, Angew. Chem. 2011, 123, 12803; Angew. Chem. Int. Ed. 2011, 50, 12595.
- 10
- 10aM. Journet, D. Cai, D. L. Hughes, J. J. Kowal, R. D. Larson, P. J. Reider, Org. Process Res. Dev. 2005, 9, 490;
- 10bY.-X. Liu, W.-M. Yan, Y.-F. Chen, J. L. Peterson, X.-D. Shi, Org. Lett. 2008, 10, 5389;
- 10cD. R. Hou, S. Alam, T. C. Kuan, M. Ramanathan, T. P. Lin, M. S. Hung, Bioorg. Med. Chem. Lett. 2009, 19, 1022.
- 11
- 11aD. Amantini, F. Fringuelli, O. Piermatti, F. Pizzo, E. Zunino, L. Vaccaro, J. Org. Chem. 2005, 70, 6526;
- 11bF. Fringuelli, D. Lanari, F. Pizzo, L. Vaccaro, Eur. J. Org. Chem. 2008, 3928;
- 11cS. Sengupta, H.-F. Duan, W.-B. Lu, J. L. Peterson, X.-D. Shi, Org. Lett. 2008, 10, 1493.
- 12For selected reviews of copper-catalyzed azide–alkyne cycloaddition reaction (CuAAC), see:
- 12aS. Díez-González, Catal. Sci. Technol. 2011, 1, 166;
- 12bL. Liang, D. Asturc, Coord. Chem. Rev. 2011, 255, 2933;
- 12cJ. E. Hein, V. V. Fokin, Chem. Soc. Rev. 2010, 39, 1302;
- 12dC. Chu, R. Liu, Chem. Soc. Rev. 2011, 40, 2177.
- 13
- 13aK. Banert, Chem. Ber. 1989, 122, 1963;
- 13bK. Banert, M. Hagedorn, Angew. Chem. 1989, 101, 1710; Angew. Chem. Int. Ed. Engl. 1989, 28, 1675;
- 13cT. Weide, S. A. Saldanha, D. Minond, T. P. Spicer, J. R. Fotsing, M. Spaargaren, J.-M. Fre‘re, C. Bebrone, K. B. Sharpless, P. S. Hodder, V. V. Fokin, ACS Med. Chem. Lett. 2010, 1, 150.
- 14An NOE analysis of 7 b demonstrates that the products of this transformation were given as the E isomer, see the Supporting Information.
- 15G. Walizei, E. Breitmaier, Synthesis 1989, 337.
- 16Bt stands for benzotriazoles. For typical applications of this versatile building block in organic synthesis, see:
- 16aA. R. Katritzky, H.-Y. Lang, J. Org. Chem. 1995, 60, 7612;
- 16bA. R. Katritzky, X.-F. Lan, J.-Z. Yang, O. V. Denisko, Chem. Rev. 1998, 98, 409;
- 16cA. R. Katritzky, A. Pastor, M. V. Voronkov, J. Heterocycl. Chem. 1999, 36, 777;
- 16dB. V. Subba Reddy, K. Praneeth, J. S. Yadav, Carbohydr. Res. 2011, 346, 995; for the synthesis and application of propargyl-1,2,3-triazoles, see:
- 16eW.-M. Yan, Q.-Y. Wang, Y.-F. Chen, J. L. Petersen, X.-D. Shi, Org. Lett. 2010, 12, 3308.
- 17L. Chen, E. Shi, Z. Liu, S. Chen, W. Wei, H. Li, K. Xu, X. Wan, Chem. Eur. J. 2011, 17, 4085.
- 18
- 18aD. R. Buckle, Encyclopaedia of Reagent for Organic Synthesis, Vol. 3 (Ed.: ), Wiley, Chichester, 1995, p. 1699;
- 18bD. Walker, J. D. Hiebert, Chem. Rev. 1967, 67, 153;
- 18cY.-Z. Li, B.-J. Li, X.-Y. Lu, S. Lin, Z.-J. Shi, Angew. Chem. 2009, 121, 3875; Angew. Chem. Int. Ed. 2009, 48, 3817;
- 18dY. Zhang, C.-J. Li, Angew. Chem. 2006, 118, 1983; Angew. Chem. Int. Ed. 2006, 45, 1949;
- 18eD. Cheng, W. Bao, Adv. Synth. Catal. 2008, 350, 1263.
- 19A similar SET oxidation mechanism for a reaction in the absence of iron catalyst was proposed in the Supporting Information as mentioned in the literature, see: Y.-H. Zhang, C.-J. Li, J. Am. Chem. Soc. 2006, 128, 4242.