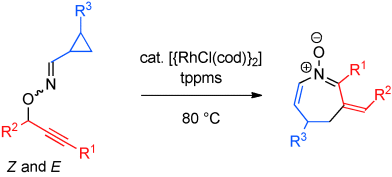
Synthesis of Azepine Derivatives by Rhodium-Catalyzed Tandem 2,3-Rearrangement/Heterocyclization†
Corresponding Author
Prof. Dr. Itaru Nakamura
Research and Analytical Center for Giant Molecules, Graduate School of Science, Tohoku University, Sendai, 980-8578 (Japan) http://www.orgreact.sakura.ne.jp/en-index.html
Research and Analytical Center for Giant Molecules, Graduate School of Science, Tohoku University, Sendai, 980-8578 (Japan) http://www.orgreact.sakura.ne.jp/en-index.htmlSearch for more papers by this authorMasashi Okamoto
Department of Chemistry, Graduate School of Science, Tohoku University (Japan)
Search for more papers by this authorYoshinori Sato
Department of Chemistry, Graduate School of Science, Tohoku University (Japan)
Search for more papers by this authorProf. Dr. Masahiro Terada
Research and Analytical Center for Giant Molecules, Graduate School of Science, Tohoku University, Sendai, 980-8578 (Japan) http://www.orgreact.sakura.ne.jp/en-index.html
Search for more papers by this authorCorresponding Author
Prof. Dr. Itaru Nakamura
Research and Analytical Center for Giant Molecules, Graduate School of Science, Tohoku University, Sendai, 980-8578 (Japan) http://www.orgreact.sakura.ne.jp/en-index.html
Research and Analytical Center for Giant Molecules, Graduate School of Science, Tohoku University, Sendai, 980-8578 (Japan) http://www.orgreact.sakura.ne.jp/en-index.htmlSearch for more papers by this authorMasashi Okamoto
Department of Chemistry, Graduate School of Science, Tohoku University (Japan)
Search for more papers by this authorYoshinori Sato
Department of Chemistry, Graduate School of Science, Tohoku University (Japan)
Search for more papers by this authorProf. Dr. Masahiro Terada
Research and Analytical Center for Giant Molecules, Graduate School of Science, Tohoku University, Sendai, 980-8578 (Japan) http://www.orgreact.sakura.ne.jp/en-index.html
Search for more papers by this authorThis work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas “Molecular Activation Directed toward Straightforward Synthesis” from the Ministry of Education, Culture, Sports, Science and Technology (Japan).
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201205285_sm_miscellaneous_information.pdf5.2 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aP. A. Wender, F. C. Bi, G. G. Gamber, F. Gosselin, R. D. Hubbard, M. J. C. Scanio, R. Sun, T. J. Williams, L. Zhang, Pure Appl. Chem. 2002, 74, 25–31;
- 1bM. Fujiwara, I. Ojima in Modern Rhodium-Catalyzed Organic Reaction (Ed.: ), Wiley-VCH, Weinheim, 2005, pp. 129–148;
10.1002/3527604693.ch7 Google Scholar
- 1cN. Jeong in Modern Rhodium-Catalyzed Organic Reaction (Ed.: ), Wiley-VCH, Weinheim, 2005, pp. 215–239;
- 1dJ. E. Robinson in Modern Rhodium-Catalyzed Organic Reaction (Ed.: ), Wiley-VCH, Weinheim, 2005, pp. 241–262;
- 1eP. A. Wender, G. G. Gamber, T. J. Williams in Modern Rhodium-Catalyzed Organic Reaction (Ed.: ), Wiley-VCH, Weinheim, 2005, pp. 263–299.
- 2
- 2aM. Murakami, K. Itami, Y. Ito, Angew. Chem. 1995, 107, 2943–2946; Angew. Chem. Int. Ed. Engl. 1995, 34, 2691–2694;
- 2bM. Murakami, M. Ubukata, K. Itami, Y. Ito, Angew. Chem. 1998, 110, 2362–2364;
10.1002/(SICI)1521-3757(19980817)110:16<2362::AID-ANGE2362>3.0.CO;2-X Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2248–2250;10.1002/(SICI)1521-3773(19980904)37:16<2248::AID-ANIE2248>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 2cM. Murakami, K. Itami, Y. Ito, J. Am. Chem. Soc. 1997, 119, 7163–7164;
- 2dM. Murakami, K. Itami, Y. Ito, Angew. Chem. 1998, 110, 3616–3619;
10.1002/(SICI)1521-3757(19981217)110:24<3616::AID-ANGE3616>3.0.CO;2-F Web of Science® Google ScholarAngew. Chem. Int. Ed. 1998, 37, 3418–3420;10.1002/(SICI)1521-3773(19981231)37:24<3418::AID-ANIE3418>3.0.CO;2-R CAS PubMed Web of Science® Google Scholar
- 2eM. Murakami, R. Minamida, K. Itami, M. Sawamura, Y. Ito, Chem. Commun. 2000, 2293;
- 2fP. H. Lee, K. Lee, Y. Kang, J. Am. Chem. Soc. 2006, 128, 1139;
- 2gX. Li, M. Zhang, D. Shu, P. J. Robichaux, S. Huang, W. Tang, Angew. Chem. 2011, 123, 10605–10608; Angew. Chem. Int. Ed. 2011, 50, 10421–10424;
- 2hX. Li, S. Huang, C. M. Schienebeck, D. Shu, W. Tang, Org. Lett. 2012, 14, 1584–1587;
- 2iS. Huang, X. Li, C. L. Lin, I. A. Guzei, W. Tang, Chem. Commun. 2012, 48, 2204–2206.
- 3
- 3aJ.-C. Guillemin, J.-M. Denis, Tetrahedron 1988, 44, 4431–4446;
- 3bS.-F. Chen, E. Ho, P. S. Mariano, Tetrahedron 1988, 44, 7013–7026.
- 4For representative reports on catalytic heterocyclization through oxidative cyclization with carbon–carbon and carbon–nitrogen multiple bonds by an aza-metallacyclic intermediate, see:
- 4aM. S. Sigman, B. E. Eaton, J. Org. Chem. 1994, 59, 7488;
- 4bT. Morimoto, N. Chatani, S. Murai, J. Am. Chem. Soc. 1999, 121, 1758–1759;
- 4cN. Chatani, T. Morimoto, A. Kamitani, Y. Fukumoto, S. Murai, J. Organomet. Chem. 1999, 579, 177;
- 4dA. Kamitani, N. Chatani, T. Morimoto, S. Murai, J. Org. Chem. 2000, 65, 9230–9233;
- 4eP. A. Wender, T. M. Pedersen, M. J. C. Scanio, J. Am. Chem. Soc. 2002, 124, 15154–15155;
- 4fC. Mukai, T. Yoshida, M. Sorimachi, A. Odani, Org. Lett. 2006, 8, 83–86;
- 4gR. T. Yu, R. K. Friedman, T. Rovis, J. Am. Chem. Soc. 2009, 131, 13250–13251.
- 5For azarhodacycles, see:
- 5aV. M. Williams, J. R. Kong, B. J. Ko, Y. Mantri, J. S. Brodbelt, M.-H. Baik, M. J. Krische, J. Am. Chem. Soc. 2009, 131, 16054–16062;
- 5bA. Dauth, J. A. Love, Angew. Chem. 2012, 124, 3694–3697;
10.1002/ange.201107669 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3634–3637.
- 6
- 6aI. Nakamura, D. Zhang, M. Terada, J. Am. Chem. Soc. 2010, 132, 7884–7886;
- 6bI. Nakamura, D. Zhang, M. Terada, J. Am. Chem. Soc. 2011, 133, 6862;
- 6cI. Nakamura, T. Araki, D. Zhang, Y. Kudo, E. Kwon, M. Terada, Org. Lett. 2011, 13, 3616–3619;
- 6dI. Nakamura, D. Zhang, M. Terada, Tetrahedron Lett. 2011, 52, 6470–6472;
- 6eI. Nakamura, Y. Kudo, T. Araki, D. Zhang, E. Kwon, M. Terada, Synthesis 2012, 1542–1550.
- 7
- 7aD. Shu, X. Li, M. Zhang, P. J. Robichaux, W. Tang, Angew. Chem. 2011, 123, 1382–1385; Angew. Chem. Int. Ed. 2011, 50, 1346–1349;
- 7bH. Gao, J. Zhang, Chem. Eur. J. 2012, 18, 2777–2782.
- 8For representative reports on rhodium-catalyzed reactions through the ring-expansion of a metal–carbinyl cyclopropane intermediate, see:
- 8aM. A. Huffman, L. S. Liebeskind, J. Am. Chem. Soc. 1991, 113, 2771–2772;
- 8bM. A. Huffman, L. S. Liebeskind, J. Am. Chem. Soc. 1993, 115, 4895–4896;
- 8cP. A. Wender, H. Takahashi, B. Witulski, J. Am. Chem. Soc. 1995, 117, 4720–4721;
- 8dP. A. Wender, C. O. Husfeld, E. Langkopf, J. A. Love, J. Am. Chem. Soc. 1998, 120, 1940–1941;
- 8eP. A. Wender, A. J. Dyckman, C. O. Husfeld, D. Kadereit, J. A. Love, H. Rieck, J. Am. Chem. Soc. 1999, 121, 10442–10443.
- 9Preparation of 1: Condensation of propargyloxyamine and cyclopropanecarbaldehyde afforded a roughly 1:1 mixture of E/Z isomers, which were readily separated by silica gel column chromatography (see the Supporting Information for details).
- 10B. Richter, A. L. Spek, G. von Koten, B.-J. Deelman, J. Am. Chem. Soc. 2000, 122, 3945–3951.
- 11Attempts to separate 2 a from triphenylphosphine oxide using flash silica gel column chromatography, GPC, or HPLC were unsuccessful.
- 12CCDC 890237 (2 a) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 13S. Ahrland, J. Chatt, N. R. Davies, A. A. Williams, J. Chem. Soc. 1958, 276–288.
- 14The reaction of (Z)-1 a in toluene at 80 °C for 24 h, in the absence of Rh catalysts, afforded 3 a (11 %) along with the recovery of 83 % of unreacted (Z)-1 a.
- 15A 1:1 mixture of the diastereomers (at the propargylic moiety) of (Z)-1 j was used. Moreover, a trace amount of the cis diastereomer (of the cyclopropane ring) was obtained in (Z)-1 j. See the Supporting Information.
- 16
- 16aM. D. Surman, R. H. Hutchings in Science of Synthesis, Vol. 17 (Ed.: ), Thieme, Stuttgart, 2004, pp. 749–823;
- 16b“Monocyclic Azepines”: G. R. Proctor, J. Redpath in The Chemistry of Heterocyclic Compounds (Ed.: ), Wiley, Chichester, 1996.
- 17For π-acidic metal-catalyzed reactions synthesizing azepine derivatives, see:
- 17aN. D. Shapiro, F. D. Toste, J. Am. Chem. Soc. 2008, 130, 9244–9245;
- 17bH. Liu, X. Li, Z. Chen, W.-X. Hu, J. Org. Chem. 2012, 77, 5184–5190;
- 17cY. Karibe, H. Kusama, N. Iwasawa, Angew. Chem. 2012, 124, 6318–6322;
10.1002/ange.201201505 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6214–6218.